
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
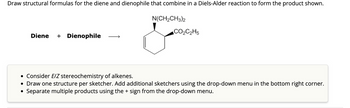
Transcribed Image Text:Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown.
Diene + Dienophile
N(CH₂CH3)2
CO₂C₂H5
• Consider E/Z stereochemistry of alkenes.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Separate multiple products using the + sign from the drop-down menu.
●
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- d Draw the major product(s) of the following reactions including stereochemistry when it is appropriate. CH₂CH₂-CEC-CH3 + 1 Cl₂ • Consider E/Z stereochemistry of alkenes. • If there is more than one major product possible, draw all of them. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ●arrow_forwardDraw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. lo Diene + Dienophile CAM • Consider E/Z stereochemistry of alkenes. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. Ⓡ Mill ChemDoodleⓇ Y -CI Sn [F CN < 46arrow_forwardDiels–Alder reaction of a monosubstituted diene (such as CH2=CH–CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO)gives a mixture of products, but the 1,2-disubstituted product oftenpredominates. Draw the resonance hybrid for each reactant, and use thecharge distribution of the hybrids to explain why the 1,2-disubstitutedproduct is the major product.arrow_forward
- Need some help filling in the missing starting materials and products. Possibly dinophile, diene, or diels alder products.arrow_forwardto form the product shown. Diene + Dienophile -> ● al CH3O CN CN . Consider E/Z stereochemistry of alkenes. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ?arrow_forwarddo all part with 30 min plz with clear handwriting in white Pagearrow_forward
- The two alkenes below react with HI at different rates. CH3CH=CHCH3 and CH₂=CH₂ Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. TAYY [ ] در ChemDoodle Ⓡarrow_forwardStep 6: Now that you have determined the substrates and mechanism of a Diels-Alder reaction, you will learn how to recognize when you should use the Diels-Alder reaction. In a synthesis reaction, if you are given a cyclohexene product with no other obvious functional group transformations and an electron-withdrawing group two carbons away from the alkene, it is likely made via the Diels-Alder reaction. Deduce the structures of the starting materials to form the Diels-Alder adduct shown. ..... CN CN Diene + Dienophilearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY