
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
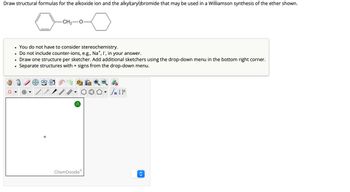
Transcribed Image Text:**Title: Structural Formula Representation in Williamson Ether Synthesis**
**Objective:**
Draw the structural formulas for the alkoxide ion and the alkyl(aryl)bromide utilized in a Williamson synthesis of the ether shown.
**Chemical Structure Provided:**
- The diagram illustrates the ether product with a phenyl group (benzene ring) bonded to an oxygen (O) and connected further by an alkyl chain CH₂ to another part of the molecule.
**Instructions:**
- **Stereochemistry Consideration:** Not required.
- **Counter-ions:** Do not include; for example, Na⁺, I⁻, etc.
- **Structure Drawing:** Use one sketcher per structure. Additional sketchers can be added via the drop-down menu in the bottom right corner.
- **Structure Separation:** Utilize the '+' sign from the drop-down menu to separate structures.
**Sketcher Toolbar:**
- A variety of tools for drawing included in ChemDoodle:
- Bond creation
- Atom label
- Shape drawing
- Manipulation tools
- Click to choose tools and sketch the intended structures in the provided area.
**Diagram Explanation:**
The starting structure for your task includes a phenyl ring connected through an ether linkage (O-CH₂) to the rest of the molecule. Your job is to identify the alkoxide and the alkyl(aryl)bromide that could form this ether through Williamson synthesis.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown.arrow_forwardDraw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. CH3 0 CH3CH₂-O-CHCH3 • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na*, I, in your answer. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu. 16 90-85 +▾ ...ll ChemDoodleⓇ Q On [Farrow_forward
- Draw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. CH3OH CH3CHCCH₂CH3 CH3 You do not have to consider stereochemistry. If there is more than one combination, draw only one. Draw one structure per sketcher. Add additional sketchers using the dropdown menu in the bottom right corner. Separate structures with + signs from the dropdown menu.arrow_forwardDraw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. CH3 CH3CH2CCH2CH3 ОН • You do not have to consider stereochemistry. • If there is more than one combination, draw only one. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.arrow_forwardDraw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. CH3 CH3CH₂-O-CCH₂CH3 CH3 You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na*, I, in your answer. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right **** corner. Separate structures with + signs from the drop-down menu. /n [Farrow_forward
- 5. Homework Helparrow_forwardDraw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. OH CH₁₂ CH2CH2CHCH3 You do not have to consider stereochemistry. If there is more than one combination, draw only one. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu. Previous Nex ChemDoodlearrow_forwardDraw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. H3C. H3C1 -CH₂OH . You do not have to consider stereochemistry. If there is more than one combination, draw only one. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. . Separate multiple reactants using the + sign from the drop-down menu. ChemDoodleⓇ Sn [Farrow_forward
- Draw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. ● H3C Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Do not include counter-ions, e.g., Na+, I-, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. ● 10-CH₂CH₂CH3arrow_forwardDraw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. CH3 I CH₂-O-CHCH3 • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na*, I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu. 85 ▾ ChemDoodle 4 ۴ ] کرarrow_forwardDraw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. CH3 CH3 CH3CHCH₂CH₂-O-CCH3 Submit Answer • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na+, I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu Separate structures with + signs from the drop-down menu. SAVIL ? ChemDoodleⓇ CH3 Retry Entire Group #[ ] در ▼n lew opies] 9 more group attempts remaining the bottom right corner.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY