
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
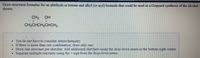
Transcribed Image Text:Draw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol
shown
111
CH3 OH
CH3CHCH2CHCH3
. You do not have to consider stereochemistry
If there is more than one combination, draw only one.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Separate multiple reactants usıng the
111
sign from the drop-down menu.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1A. Explain why solvents such as anhydrous diethyl ether or anhydrous tetrahydrofuran (THF) are not reactive with Grignard reagents. 1B. Draw the first step of the reaction mechanism when the Grignard reagent attacks benzophenone before the alkoxide is protonated to form the alcohol. In the mechanism, identify the partially positive and partially negative atoms in the reactants. Explain why these atoms have a partial charge. 1C. Write the mechanism for the reaction of phenyl magnesium bromide with 1) water and 2) acetone.arrow_forwardDraw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. CH3OH CH3CHCCH₂CH3 CH3 You do not have to consider stereochemistry. If there is more than one combination, draw only one. Draw one structure per sketcher. Add additional sketchers using the dropdown menu in the bottom right corner. Separate structures with + signs from the dropdown menu.arrow_forward29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forward
- O Predict the major monobromination product for each reaction. Draw the line angel structure and give its IUPAC name. UV light a. methylcylcopentane + Br2 UV light b. (CH3) CH2 C(CH3)2 CH2CH3 + Br2 UV light c. CH (CH3)2 CH2 CH3 + Br2arrow_forwardQuestion #9arrow_forwardState quick predictions for NaI/Acetone Test (Sn2) for each compound: 2-Bromobutane 1-bromobutane 2-Chlorobutane 1-chlorobutane t-butyl chloride benzyl chloride bromobenzene bromocyclohexane bromocyclopentanearrow_forward
- Draw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a Grignard synthesis of the alcohol shown. CH3 CH3CH2CCH2CH3 ОН • You do not have to consider stereochemistry. • If there is more than one combination, draw only one. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.arrow_forwardplease draw 3d structures (wedge and dash)arrow_forwardHELP MEarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY