
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
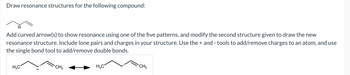
Transcribed Image Text:Draw resonance structures for the following compound:
Add curved arrow(s) to show resonance using one of the five patterns, and modify the second structure given to draw the new
resonance structure. Include lone pairs and charges in your structure. Use the+ and - tools to add/remove charges to an atom, and use
the single bond tool to add/remove double bonds.
H₂C
CH₂
H3C
CH2₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining resonance structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding Structure B: draw the remaining resonance structure, nonbonding electrons and formal charges. including nonbonding electrons and formal charges. Rings Erase Select Draw Rings More Erase Select Draw More H // C H. Harrow_forwardAdd lone pairs to the central atoms as necessary to complete the Lewis structures. Do not worry about the positions of the lone pairs on the central atom. Focus on the number of lone pairs. XEF2 SF, Erase Select Draw Rings More Erase Select Draw Rings More S F Xe Xe F :arrow_forwardNext, add curved arrow(s) to show the resonance using the following pattern: a lone pair adjacent to C+. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to increase or decrease the charge on an atom, and use the single bond tool to add/remove double bonds. :0 :: NH₂ Edit Drawing H NH₂arrow_forward
- Show three or four different drawings that show three or four different relevant resonance structures for the compound shown below. Be careful to use correct arrows when that show electron movements between each resonance shown Also, draw the hybrid. NH Oarrow_forwardPlease provide only typed answer solution no handwritten solution neededarrow_forwardBC3 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. An H N F Br X Morearrow_forward
- A compound composed of 3.3% H, 19.3% C, and 77.4% O has a molar mass of approximately 60 g/mol. What is the molecular formula of the compound? molecular formula: НСО Draw the Lewis structure of the compound where the H atom(s) are bonded to O atom(s). Select Draw Rings More Erase What is the geometry around the C atom in this compound?arrow_forwardDraw any resonance structures for the following molecule. OH HO Draw all possible resonance structures by copying the skeleton shown. Edit bonds and charges to complete each resonance structure.arrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atom carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. H₂C: a H₂ Submit Answer O -Η The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is Retry Entire Group 1 more group attempt remainingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY