
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
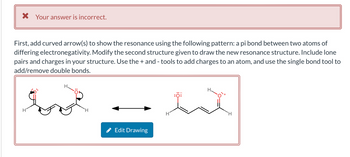
Transcribed Image Text:X Your answer is incorrect.
First, add curved arrow(s) to show the resonance using the following pattern: a pi bond between two atoms of
differing electronegativity. Modify the second structure given to draw the new resonance structure. Include lone
pairs and charges in your structure. Use the + and - tools to add charges to an atom, and use the single bond tool to
add/remove double bonds.
H
H
Edit Drawing
H
:0:
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the relationship between the following compounds? A. constitutional isomers B. different representations of the same molecule C. different molecules D. isotopes E. resonance structuresarrow_forward+] QUESTION 20 Which is true regarding the H-F bond in the compound HF it is ionic O it is polar covalent it is nonpolar covalent none of the above QUESTION 21 Explain your reasoning for the previous problem For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). Paragraph Arial 10pt Because Hydrogen fluoride is a gas and doesn't form a solids ionic compounds. P. Click Save and Submit to save and submit. Click Save All Answers to save all answers. M.arrow_forwardBC3 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. An H N F Br X Morearrow_forward
- Steps for Lewis Structures: 1. Determine the total number of valence electrons. Add electrons for negative charges, subtract electrons for positive charges. 2. Place least electronegative atom (except H) as central atom in structure. 3. Connect atoms by singles bonds. Each single bond = 2 electrons. 4. “Sprinkle” remaining electrons around outside atoms first to complete octets. Don’t use more electrons than the total found in step 1. Then complete the central atom’s octet last if you have enough electrons. 5. Make double or triple bonds as needed to complete octets. 6. Place brackets and charge for ions. For the central atom in each formula, draw the Lewis Structure with all valence electrons shown. 1. PH3 2. H2O 3. CO2 4. CHCl3 5. O2 6. N2 7. CF4 8. C3H8 9. CH3COOH 10. N2O 11. OCN-arrow_forwardNonearrow_forwardQUESTION 3. Label each bond with &t or & to show the direction of polarity. You can just type your answers as either (+) or (-) instead of using the Greek symbols above if you have a hard time looking for them in MS Word's "Symbols." C-O Si----C C- F Be----Cl F-----Cl S- Oarrow_forward
- A compound composed of 3.3% H, 19.3% C, and 77.4% O has a molar mass of approximately 60 g/mol. What is the molecular formula of the compound? molecular formula: НСО Draw the Lewis structure of the compound where the H atom(s) are bonded to O atom(s). Select Draw Rings More Erase What is the geometry around the C atom in this compound?arrow_forwardPart A Review | Constants | Periodic Table Draw a resonance contributor for HOCO₂. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all hydrogen atoms. Include all lone pairs of electrons. Show the formal charges of all nonhydrogen atoms in the correct structure. ? Q iarrow_forwardHelp!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY