
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
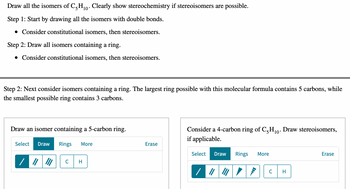
Transcribed Image Text:Draw all the isomers of C5H₁0. Clearly show stereochemistry if stereoisomers are possible.
Step 1: Start by drawing all the isomers with double bonds.
• Consider constitutional isomers, then stereoisomers.
Step 2: Draw all isomers containing a ring.
• Consider constitutional isomers, then stereoisomers.
Step 2: Next consider isomers containing a ring. The largest ring possible with this molecular formula contains 5 carbons, while
the smallest possible ring contains 3 carbons.
Draw an isomer containing a 5-carbon ring.
Rings More
Select Draw
/
C
H
Erase
Consider a 4-carbon ring of C5H₁0. Draw stereoisomers,
if applicable.
Select Draw Rings
More
C
H
Erase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- COOEt COOEt 1. Eto Na+ 2. HCI, H₂O compound a NaOH, H₂O Heat • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Do not include counter-ions, e.g., Na+, I, in your answer. compound b HCI, H₂O Heat Claisen condensation between a diethyl ester and an ethyl ester followed by saponification, acidification, and decarboxylation forms a diketone. Work out this synthesis on a separate sheet of paper, and then draw the structure of compound a. compound c (C9H12O2)arrow_forwardDraw a structural formula for 2,3,3-trimethylhexanoic acid. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. ... ? ChemDoodleⓇ laarrow_forward3. Draw Newman projection for the highest and lowest energy conformers of your assigned molecule. Provide a rationale to support your answer. In other words, why is the highest energy conformer so high in energy? Why is the lowest energy conformer low in energy? Molecule (2R,3S)-2-Bromo-3- Floro butanearrow_forward
- Draw the structure of the following molecules. Molecules are given below in the images please draw there strarrow_forwardOH If the above molecule is molecule A, and is in a 50/50 mixture with molecule B, the mixture would be deemed racemic. Draw molecule B. What is the relationship between molecule A and B?arrow_forward2. For the following nucleophilic substitution reaction: NaOC(CH3)3 CH3 'CH3 CH3 a. Draw the first transition state (TS1), the reaction intermediate, and the second m transition state (TS2) in the labeled boxes. Make sure to show any formal charges, unfilled p-orbitals, and correct stereochemistry. TS₁ 41001 Intermediate ogmos TS2 amond- b. Which of three molecules you drew for part 'a' is lowest in energy? Write your answer in the box below. c. Draw the product(s) of the nucleophilic addition reaction shown. Make sure to use wedged and dashed lines to clearly show the resulting stereochemistry at each chiral center. If there is more than one possible product, draw one in each box - if not, leave one box blank. CH3 Br + NaOC(CH3)3arrow_forward
- Can you please please help me with these problems. Thank you so much!arrow_forward| 16. Which conformation of cyclohexane experiences the most transannular strain? a. chair b. planar c. boat d. twist boat e. All of these are stable. basdarrow_forward6. For each of the following, draw the most stable chair form and identify whether the more stable stereoisomer would be the cis or the trans stereoisomer? a) trans 3-tert-butyl-1-methylcyclohexane b) Cis-2-chloro-1-ethylcyclohexane c) trans-3-butyl-1-isopropylcyclohexane d) trans-4-hydroxy-1-t-butylcyclohexane چارarrow_forward
- 3. Draw the bond line structure that corresponds to the names of the following compounds: a) (S)-6-chloro-1-methylcyclohexa-1,4-diene. b) (S)-hept-5-yn-2-olarrow_forwardCar note Pirate Ship BLACKBOARD [Review Topics] [References) Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. CH3 HạC CH3 H3C CI Submit Answer Retry Entire Group 9 more group attempts remaining Next Previous Save and Exit étv 14 MacBook Air 12 F11 F9 888 FB F7 F5 F4 & $ % 8 9 6 7 4 Y U R F G H. ? N M V .. ..arrow_forwardDraw two cyclic constitutional isomers of trans-1,2-dimethylcyclopentane with the same size ring. • In cases where there is only one cyclic constitutional isomer, just draw one structure. • If there are more than two isomers, draw any two of the possible structures. • Your drawing must specify cis/trans stereochemistry unambiguously using wedged and dashed bonds. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ? ChemDoodle it'sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY