
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Draw a structural formula for the major product of the acid-base reaction shown.
- You do not have to consider stereochemistry.
- Do not include counter-ions, e.g., Na+, I-, in your answer.
- In those cases in which there are two reactants, draw only the product from the compound that reacts.
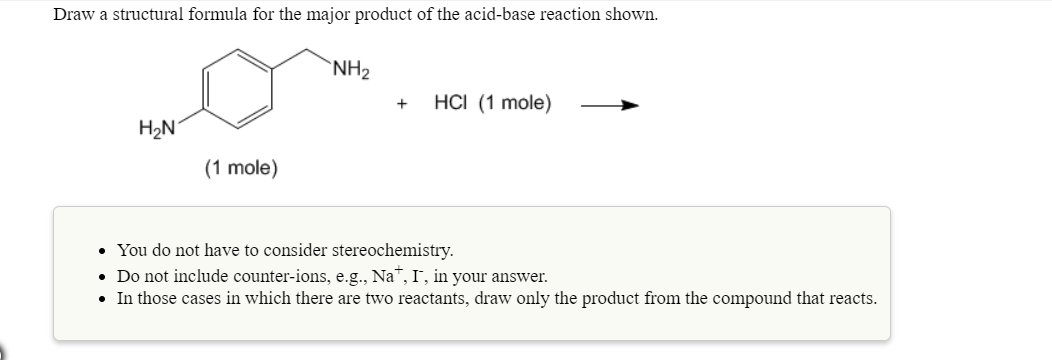
Transcribed Image Text:Draw a structural formula for the major product of the acid-base reaction shown.
NH2
HCl (1 mole)
-
H2N
(1 mole)
. You do not have to consider stereochemistry
Do not include counter-ions, e.g., Na, I, in your answer.
In those cases in which there are two reactants, draw only the product from the compound that reacts
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please helparrow_forwardPropose a structure for an aromatic hydrocarbon, C3H10, that can form three C3H,Br products on substitution of a hydrogen on the aromatic ring with bromine. • You do not have to consider stereochemistry. In cases where there is more than one answer, just give one. • Ignore the ortho, para-directing effects of the alkyl groups in answering this question. Consider only the number of nonequivalent hydrogens on the aromatic ring. .... ChemDoodlearrow_forwardThe S 1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is the rapid reaction of the carbocation intermediate with the nucleophile; this step, completes the nucleophilic substitution stage. The step that follows the nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal charges. Draw or edit atoms, groups, or bonds Add/Remove step 2 9 Click and drag to start drawing a structure. alearrow_forward
- 2. Assuming products derive from the MORE stable carbocation intermediate, draw the organic product(s) you would expect from the following reactions. CH3 + HCI (1 mole) + HBr (1 mole) + HBr (1 mole)arrow_forwardQUESTION 2a between one equivalent of HBr with the compound shown below? What would be the major 1,2 and 1,4 products formed by the reaction Indicate which is the kinetic and which is the thermodynamic product and the reasoning for your choice (1-2 sentences maximum). Draw an energy diagram to help explain your choice. intermediates. Also, how would it is possible to favor formation of the 1,4 over 1,2 product and the Show the complete arrow pushing mechanism for the formation of each product and all 1,2 over 1,4 product. HBr (1 eq)arrow_forwardDraw the major organic product(s) of the following reaction. CI H₂O + NaOH You do not have to consider stereochemistry. •If no reaction occurs, draw the organic starting material. • When SN1 & E1 pathways compete, show both the substitution and the elimination products. • Separate multiple products using the + sign from the drop-down menu. Do not include counter-ions, e.g., Na+, I, in your answer.arrow_forward
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forward(11) Draw the major organic product of the reaction. Follow this procedure: determine whether the reaction conditions are acidic or basic; identify the most nucleophilic/basic atom, the electrophilic atom, and the leaving group; predict whether elimination or substitution will occur; and then draw the product. Indicate the stereochemistry at every stereocenter with a single wedged (up), hashed (down), or wavy (a mixture of up and down; either) bond. Launch Marvin JS TM viewer or click image to copy source HN CI Ο N-Karrow_forwardDraw a structural formula for the more stable carbocation intermediate formed in the reaction shown. o =CHCH₂CH3 + HI • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • For cases in which carbocations of the same or similar stability are expected, draw all of the structures. • Draw one structure per sketcher. Add additional sketchers using the drop- down menu in the bottom right corner. Separate structures with + signs from the drop-down menu.arrow_forward
- NaOH (i). .Br NaH (). ofarrow_forwardDraw a structural formula for the more stable carbocation intermediate formed in the reaction shown. CH3 H2C=CHCH2CHCH3 + HI • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I", in your answer. • For cases in which carbocations of the same or similar stability are expected, draw all of the structures. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. P aste opy ChemDoodlearrow_forwardDraw a structural formula for the more stable carbocation intermediate formed in the reaction shown. CH3CH2 CH₂CH₂CH3 H H + HI • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • For cases in which carbocations of the same or similar stability are expected, draw all of the structures. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down me menu. ChemDoodleⓇ [ ] در > Previous Ne:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY