
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
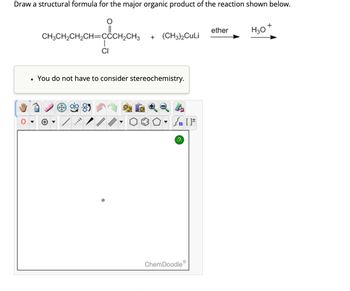
Transcribed Image Text:Draw a structural formula for the major organic product of the reaction shown below.
+
ether
H3O
CH3CH2CH2CH=CCCH2CH3
+ (CH3)2CuLi
CI
•
You do not have to consider stereochemistry.
?
ChemDoodleⓇ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the expected major products, X & Y, upon reaction of HBr with the alkene shown under two different conditions indicated? HBr, ROOR Y HBr heat Br Br Br II Major productX= choose your answer. ^ Major product Y= choose your answer... choose your answer... II IIarrow_forwardanswer does not include dashes or wedgesarrow_forwardb. Increasing reactivity with HBr H3C Н H3C A Н least reactive н н Н В I H3C Н C I н н Н D CH₂CH3 most reactivearrow_forward
- An alkene having the molecular formula C,H12 is treated sequentially with ozone (O3) and zinc/acetic acid to give the product/s shown. CH3CH2CH,CH½CH2CH Draw a structural formula for the alkene. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. P opy aste Previous Nextarrow_forwardDraw the structure of the major organic product of the reaction below. CH3 CH₂CHCH₂CH₂CH=CH₂ + CHC13 KOH • Use the wedge/hash bond tools to indicate stereochemistry where it exists. Show stereochemistry in a meso compound. If the reaction produces a racemic mixture, just draw one stereoisomer.arrow_forwardDraw the four major products that form, including stereoisomers, in the bromination of cyclopentene with Br2 and light. Make sure you draw all four bonds at all stereocenters. Br₂ hyarrow_forward
- A E OH OH H This is a(n) OH R B F PCC, CH₂Cl₂ H type reaction. The major product if R = CH₂CH3 is compound The major product if R = H is compound G Look at the given reaction and use the letter code corresponding to each compound in the blank to indicate the expected product(s), or fill in the blank with the appropriate vocabulary word or phrase. If a specific stereoisomer (e.g single enantiomer) is formed, select that isomer only. If a mixture of stereoisomers (e.g. racemic or diastereomers) is formed, select the single structure that shows the appropriate mixture (e.g. lines not dashes). If we convert the reagent to Na2CrO4, H₂SO4, H₂O: The major product if R = CH₂CH3 is compound The maior product if R = H is compound ??? OH D H H though compoundarrow_forward5. Which bromocyclohexane starting material would react faster - the cis or trans, and explain why? Draw both chair forms of the starting material to prove your point. Br K* OC(CH) K* OC(CH); Br cis transarrow_forwardQ 10 pleasearrow_forward
- Q 13 pleasearrow_forwardOH CH3-CH2-C-O-CH2-CH3 H OH CH3-CH₂-C-OH HI CH3-CH₂-0-0-CH₂-CH3 ||| Predict the product of the following reaction. + HO-CH2-CH3 H* O-CH2-CH3 CH3-CH2-C-O-CH2-CH3 H || CH3-CH₂-C-CH₂-CH3 IV A) I B) II C) III D) IVarrow_forwardProvide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) Br. H C=C CH3 H₂C-CH₂ C=C H CHCH(CH3)2 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY