
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
i need help
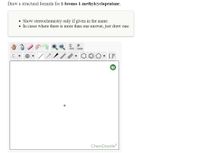
Transcribed Image Text:Draw a structural formula for 1-bromo-1-methylcyclopentane.
• Show stereochemistry only if given in the name.
• In cases where there is more than one answer, just draw one.
opy aste
ChemDoodle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This assignment is designed to help you learn how to determine total costs by combining direct and indirect costs for a particular department. In this GHA, you will be determining the total costs for three departments: (1) Physical Therapy (2) Occupational Therapy and (3) Speech Therapy. To determine the total costs, you will need to add the direct costs to the indirect costs using the template provided below. Please use the example provided in the assignment description if you find that you need some support understanding the required calculations. DIRECT COSTS The direct costs for each department are easily identifiable, because they are the costs that are directly attributable to the department. The direct costs are as follows: Physical Therapy (PT) Occupational Therapy (OT) Speech Therapy (ST) Total Direct Costs INDIRECT COSTS The indirect costs will need to be allocated to Physical Therapy (PT), Occupational Therapy (OT), and Speech Therapy (ST). The indirect costs in this…arrow_forwardI have no idea how to do the rest please helparrow_forwardResearch Project P X Research Project F X Paraphrasing Tool Why Are Healthy E X CDC Improving Your Eat X Lab Report: Soluti x Course Home New Tab openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=db2c21b62c49dad8f42838d80fceb3b7#10001 Apps Yahoo Mail YouTube Maps Best Free PowerP... Google Drive Academic Search I Downloads e University Librarie... E UNIVERSITY POR... Student Detail Sc... I Review I Constants I Periodic Table Scores Calculate the number of moles in 5.00 g of each of the following: Part C eТext CS2 Document Sharing Express your answer in moles. User Settings ΑΣφ ? Course Tools > n = mole Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Starting with 5.00 g of CS2, set up the calculation so that the molar mass of CS2 is a conversion factor where the unwanted unit of grams cancels out and the desired unit mole remains. Part D CazN2 Express your answer in moles. ? n = mol Submit Request Answer P Pearson Copyright © 2021…arrow_forward
- Can i get help pleasearrow_forwardCarbon dioxide concentration (parts per million) Examine the graph and answer the following questions. Part A Atmospheric Carbon Dioxide What was the average rate of increase in carbon doxide concentration between 1900 and 19407 410 400 Express you answer in parts per million per year to two significant figures. 300 380 a ? 370 360 ppm/year 350 340 Submit Bequest Anawer 330 320 310 Part 8 Complete previous parts) 300 290 - 1860 1880 1900 1920 1940 1960 1980 2000 2020 Provide Feedback Next> Yeararrow_forwardhelp mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY