
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
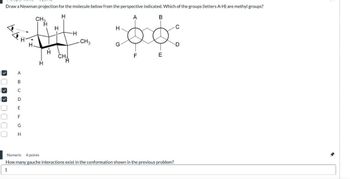
Transcribed Image Text:Draw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups?
CH3
H
H
H
A
H
B
☑
>>
H.
ABCDEFG I
H
-H
CH3
G
D
CH
F
E
Numeric 4 points
How many gauche interactions exist in the conformation shown in the previous problem?
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider the molecule 1-bromo-2-methylbutane. C3 and C4 should be drawn as Et as in theexample. This group is called an ethyl group and can be considered a sphere about twice the sizeof a methyl group. Draw the following Newman projections sighting down the C1C2 bond... a. The lowest potential energy conformation. b. The highest potential energy staggered conformation.arrow_forwardUsing your model of butane (CH3CH2CH2CH3) , complete the following graph of the anglebetween the two Me groups vs. potential energy. a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.) b. Draw a wedge and dash bond representation of butane in its lowest P.E. conformation.arrow_forwardIn the next chapter we'll look at cycloalkanes—saturated cyclic hydrocarbons—and we’ll see that the molecules generally adopt puckered, nonplanar conformations. Cyclohexane, for instance, has a puckered shape like a lounge chair rather than a flat shape. Why?arrow_forward
- Draw the most stable conformation of 1,4-dichlorobutane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.arrow_forwardUse your answers from Problem 2.48 to complete the table showing correlations between cis,trans and axial,equatorial for disubstituted derivatives of cyclohexane. Draw the alternative chair conformations for the cis and trans isomers of 1,2-dimethylcyclohexane, 1,3-dimethylcyclohexane, 1,4-dimethylcyclohexane. (a) Indicate by a label whether each methyl group is axial or equatorial.arrow_forward3. Draw both chair conformations for the cyclohexane molecule shown below, and circle the most stable conformation (the methyl group is larger than the hydroxyl group). OH H3Carrow_forward
- See image belowarrow_forwardF) Circle the letter corresponding to the relationship of each pair of structures: Identical (I), Conformers (C), Enantiomers (E) (enantiomers can have different conformations). FIRST... determine the absolute configuration of chiral centers. H. ІН' I H | H с H Me E Mearrow_forwardNonearrow_forward
- Draw a Newman projection for two more staggered conformations of this molecule. Which of your conformations is most stable? Assume that -OH and -CH3 are comparable in size.arrow_forwardNonearrow_forward6. Newman projections to Lewis structures From the following Newman projections, draw the corresponding Lewis structure in the same conformation. A) B) H. H CH3 H₂C N H OH CI Br CH3 H OH CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning