
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw a molecular orbital diagram for CN-, indicating u and g symmetry notation. Assume
σ and π order consistent with C2 and N2 diatomic molecule MO diagrams. Calculate the
bond order for CN- and determine its magnetic properties.
Expert Solution
arrow_forward
Step 1
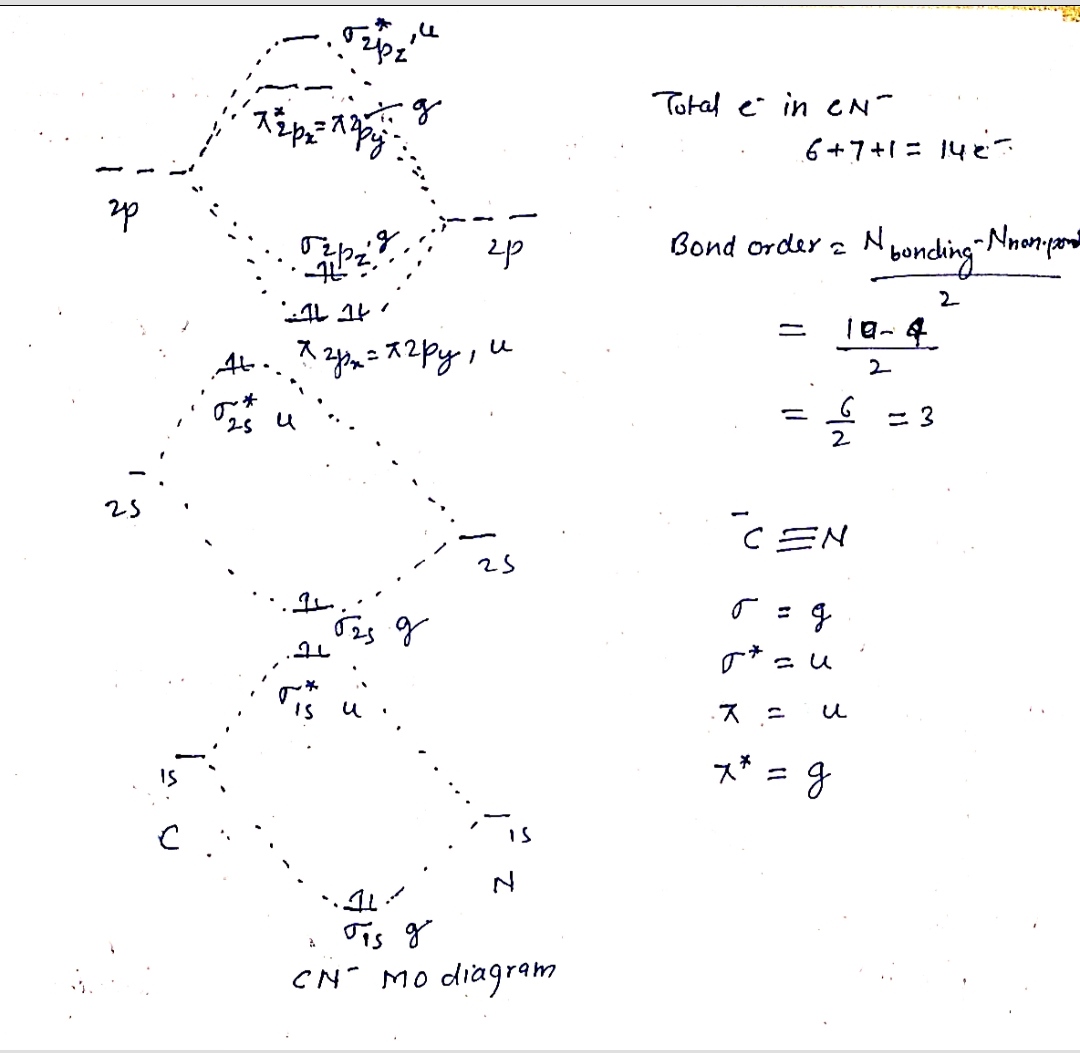
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. The following phosphorous halide molecules contain P as the central atom. i. Draw the expected molecular shape, determine whether it is polar, and indicate the overall direction of the dipole moment. (You do NOT need to show resonance or formal charges AND if there are more than one isomer, please pick one for your answer ii. List all its symmetry elements and show all unique symmetry elements that are not the identity operator or an improper rotation. iii. Determine its point group. а. POCI3 b. РОCІBrz С. РOСІBrlarrow_forwardSketch the MO diagram for 1,3 budadiene. Identify the HOMO and LUMO along with all symmetric MO’s, asymmetric MO’s, bonding MO’s, and anti bonding MO’s.arrow_forwarde. What are the characters for the irreducible representations for E"? f. If one O atom is placed in a D3h point group, what is the Mulliken symbol for its px orbital? g. If an Co²+ ion is placed in a D3h point group, what is the Mulliken symbol for its dxz orbitals? 4. One application of the Group Theory in chemistry is to predict vibrational modes of a molecule. For example, Mn(CO)5Cl (shown below) only gives one IR band. By answering the following questions, you will be able to understand why this is the case. OC-Mn- СО OC a. What is the point group of this molecule? b. Copy the Character Table for the identified point group from your textbook (Appendix C) here.arrow_forward
- Using a group theoretical approach, generate the MO diagrams for silane, SiH4. Be sure that your diagram is fully-labeled with MO assignments, is properly scaled in energy space, and provide a sketch of the MO. Determine the bond order for the silane molecule, and for the Si-H bond.arrow_forwardH-He-H2+ is a linear trinuclear with He occupying the central position and the two hydrogen occupying the terminal positions. Build a reasonable qualitative molecular orbital diagramarrow_forwardOn one-electron oxidation of a five valence electron AH₂ compound (C₂, point group) to a closed shell (no unpaired electrons) four valence electron C₂, cationic species, what is the symmetry of the highest energy occupied molecular orbital, and what happens to the energy of this orbital when the four valence electron AH2 C2y cation changes to a Doch geometry? O 2a₁ and stabilised (decreases in energy) O 2a₁ and destabilised (increases in energy) O 1b2 and stabilised (decreases in energy) O b₁ and no change in energyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY