
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
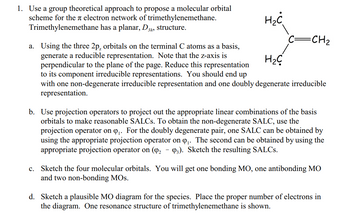
Transcribed Image Text:1. Use a group theoretical approach to propose a molecular orbital
scheme for the electron network of trimethylenemethane.
Trimethylenemethane has a planar, D3
3h, structure.
H₂C
=CH₂
a. Using the three 2p, orbitals on the terminal C atoms as a basis,
generate a reducible representation. Note that the z-axis is
perpendicular to the plane of the page. Reduce this representation
to its component irreducible representations. You should end up
H₂C
with one non-degenerate irreducible representation and one doubly degenerate irreducible
representation.
-
b. Use projection operators to project out the appropriate linear combinations of the basis
orbitals to make reasonable SALCs. To obtain the non-degenerate SALC, use the
projection operator on Q₁. For the doubly degenerate pair, one SALC can be obtained by
using the appropriate projection operator on ₁. The second can be obtained by using the
appropriate projection operator on (9₂ 93). Sketch the resulting SALCs.
c. Sketch the four molecular orbitals. You will get one bonding MO, one antibonding MO
and two non-bonding MOS.
d. Sketch a plausible MO diagram for the species. Place the proper number of electrons in
the diagram. One resonance structure of trimethylenemethane is shown.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw an mo diagram for the valence electrons in C2. What is the bond order many sigma and pi bonds are there? What is the HOMO(Highest Occupied Molecular Orbital) and LUMO(Lowest Unoccupied Molecular Orbital)? What is the magnetism of the species?arrow_forward1. Pyridine (shown below) is a planar delocalized molecule Similar to benzene H 1) Identify its principal rotation axis (draw it it on picture) 11) Determine its point group and give your resoning / explainin H H C IC H 。 H and label 1211arrow_forward6-8arrow_forward
- Draw an MO diagram to explain the existence of the trihydrogen cation (H3+). Hint: it forms a cyclic structure. Use individual 1s orbitals from H atoms to form your molecular orbitals (don’t worry about forming LGOs; determine the symmetries of the MOs using the projection operator method and sketch approximate wavefunctions for each). How many electrons are present in this molecule? What is the bond order? What type of bonding is this?arrow_forward3. Calculate the coefficients c+ and c. and energies E+ and E. for the bonding and anti- bonding H₂ Molecular orbitals. HAA = HBB = -13.6 eV and HAB = -14.3 eV. Assume that SAB = 0.3arrow_forward1. Give the point group for each of the following molecules: H H H CI F F H H. CI BrH BrH Br Br Br Br CI H. Сyclohexane (chaй) Cyclohexane (boat) Ferrocene (staggard) Ferrocene (eclisped) 2. Give the point group for each of the following atomic and molecular orbitals: 88 dy d22 o bond n bond z antibond sp3arrow_forward
- On one-electron oxidation of a five valence electron AH₂ compound (C₂, point group) to a closed shell (no unpaired electrons) four valence electron C₂, cationic species, what is the symmetry of the highest energy occupied molecular orbital, and what happens to the energy of this orbital when the four valence electron AH2 C2y cation changes to a Doch geometry? O 2a₁ and stabilised (decreases in energy) O 2a₁ and destabilised (increases in energy) O 1b2 and stabilised (decreases in energy) O b₁ and no change in energyarrow_forward3. Assign each molecule or ion to its proper point group. a. cyclopropylene b. aziridine (hint: be very mindful of lone pairs) c. NbF,2-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY