
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
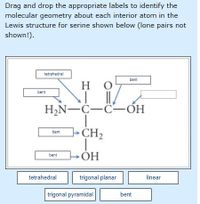
Transcribed Image Text:Drag and drop the appropriate labels to identify the
molecular geometry about each interior atom in the
Lewis structure for serine shown below (lone pairs not
shown!).
tetrahedral
bent
H
bent
H,N-C-C-ÓH
>CH2
bent
ÞOH
ОН
bent
tetrahedral
trigonal planar
linear
trigonal pyramidal
bent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of these statements concerning p-orbitals is false? 1. They consist of two equivalent lobes. 2. They are absent from the first shell of atomic orbitals. 3. They can form & bonds. 4. They only participate in bonding on carbon atoms. 5. They can hold a maximum of two electrons.arrow_forward< 8:02 A) sulfide B) disulfide What type of bond is found between two -SH groups and can be found in proteins? Question 23 of 40 C) thioether D) sulfur E) disulfur SOS 63 Tap here or pull up for additional resources Submitarrow_forwardWhich molecule contains carbon with a negative formal charge? CO CO2 H2CO CH4arrow_forward
- Click on those molecules below which have a dipole moment. If there are none, submit your answer without clicking. HC CH3 || CH₂ Н. H3C CH3 'H ه CH₂ CIarrow_forwardopposite polarities. Also Hote that for cal lydrogen bohas both carbon and hydrogen äre con Peptide *NH3 Octane Water Butanol H. CH, CH3 1. O=C CH, НС-ОН 1. HC-CH,-CH,OH H CH, CH, CH, C=0 CH, CH, CH, NH CH3 CH HC-CH,-CH,OH CH, CH C=0 CH, CH, NH CH, CH, HC-CH,-COO- CH, C=0 CH, NH CH3 HC-CH,-CH,-CH,-CH,-"NH, CH. C=0 CH, O=P-O Question 1 Lauric Acid Phosphate Derivativearrow_forwardPlease do no with students who have not yet After your first attempt, you may retake the quiz one additional time. In order to n Question 1 What is the molecular formula of the following compound? Please write your answer using this format: C25H32NO Question 2 NH₂ What orbitals overlap to form the indicated carbon-carbon bond?arrow_forward
- Identify the hybridization and bond angles at the carbon atoms in the molecule shown:arrow_forwardLabel the molecular shape around each of the central atoms in the amino acid glycine. H H H I. H :0: •H Answer Bank trigonal pyramidal bent linear trigonal planar tetrahedralarrow_forwardH-p, h-bi,h-n,h-ag,h-p least polar?arrow_forward
- Choose the correct resonance hybrid for the following compound. 0 O O 49 -O-H ●ㅗㅗ 8+ 8+ HO-H co ---- 8+ 8+ H ○- + DHE O-H 8+ 8-Harrow_forwardIndicate which of the following molecules are polar. For those that are, indicate the direction of the molecular dipole using the formalism used in the Karty text. Br NH2 CI -Ć -Br NH Farrow_forwardSecond picture is the other two answer choicesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY