
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
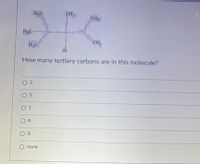
Transcribed Image Text:CH
HC-
CH
HC
How many tertiary carbons are in this molecule?
O 2
O 5
0 1
O 4
O 3
O none
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Jj.11.arrow_forwardIdentify the structure that shows the correct placement of all lone pairs for the compound illustrated in the box below. امعه H3N. H3N H3N. H3N. w to wiele weite 0: O-H O-H ||| I = OH ||| H3N. H-Ö IV H3Narrow_forwardChoose the correct resonance hybrid for the following compound. 0 O O 49 -O-H ●ㅗㅗ 8+ 8+ HO-H co ---- 8+ 8+ H ○- + DHE O-H 8+ 8-Harrow_forward
- Formal charges and bonds are shown correctly in this Lewis structure, but lone pairs might be drawn wrong. Add or remove lone pairs to fix the structure. If no corrections need to be made, please check the box below. H H H H :0-C HH C= H =N H CI There are no missing or incorrect lone pairs. P X c+ G 'U 0: C Aarrow_forwardch4 + o2 co2 + 2h2oarrow_forwardDoes H4C20 have double bonds? No. Yes, 2 double bonds. Yes, 1 double bon d. Yes, 3 double bonds Yes, 4 double bonds. O0 00arrow_forward
- 1. Draw a skeleton and make sure the bonds and lone pairs above are added. It is usually best to start with the carbon atoms, then add oxygens, then hydrogens, and fill in with lone pairs ( normally not on carbon ) or multiple bonds if necessary. С — С — С —о 2. Add the hydrogens to the carbon - oxygen skeleton. ннн H С —С | ннн 3. Complete the structure by adding lone pairs (2) to the oxygen atom. ннн H 0-H нннarrow_forwardDraw skeleton line structure. Be sure your structures show the important difference between the molecules.arrow_forwardPredict the product of this organic reaction: CH3-C-OH + CH₂-OH A →P + H₂O Specifically, in the drawing area below draw the condensed structure of P.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY