
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chem help! Urgent!
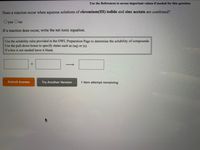
Transcribed Image Text:Use the References to access important values if needed for this question.
Does a reaction occur when aqueous solutions of chromium(III) iodide and zinc acetate are combined?
Oyes Ono
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Use the pull-down boxes to specify states such as (aq) or (s).
If a box is not needed leave it blank.
Submit Answer
Try Another Version
1 item attempt remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10:15 PM Tue Sep 12 12.0 12.0 gtt ml A patient is to receive: 600.0 mL IV fluid over 8.00 hours. The drop factor for the tubing is 12.0 gtt/mL. What is the correct flow rate setting in drops per min ? (1 drop = 1 gtt) stt mL 1 drops 1 att 8.00 ADD FACTOR x( ) 96.0 drop X 0.833 900 1 1 Question 4 of 5 DELETE 9.60 drops stt drop/gtt mL/hr 1.25 600.0 120 0.104 15.0 60 X gtt/mL ANSWER 0.0667 mL RESET 2 1 drop/min Submit 12.0 54% L 375arrow_forwardDraw the starting alkene that would lead to these major products under these conditions. Drawing H₂ Pd/C 11arrow_forwardCalculate the maximum solubility (in mol/L) of copper(1) chloride in water. The Ksp of CuCl is 1.72 x10-7 8.60 x10-8 mol/L 4.15 x10-4 mol/L 3.56 x10-2 mol/L 6.44 x 10-3 mol/Larrow_forward
- OnCourse Connect Assessment: Chemistry x Copy of spread_of_islar x = The Spread of Islam -G x M Inbox (904) - aarojame A Classes boncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4 D TPSS Bookmarks CHEMISTRY BENCHMARK TEST 01 CHEMISTRY I 12-7 (AARON JAMES, ID: 12390724) A student dissolved 15 grams of pure acetic acid (CH,COOH) in enough water to make 100. milliliters of solution. Given the atomic masses provided on the periodic table, what is the molarity (M) of the acetic acid solution? a 0.025 molar D0.40 molar © 2.5 molar O 4 molar A B B 1 2 4 5 6 7 8 9 10 3. O Save/Exit 4 sn TESSarrow_forward2. An 84 kg University of Toledo groundskeeper who is spraying a 100 mg/L solution of the insecticide dieldrin (MW = 381; logP = 4.56) on some trees exposes 100 cm“ of his skin to the solution over a 4 hr period. Dieldrin has a half-life of about 6 months, a volume of distribution of about 40 L/kg, and a dermal permeability of about 3 x 10° cm/sec. (a) Estimate the absorption rate during the exposure period assuming the blood concentration is much lower than the skin concentration (i.e., take the blood level to be zero in the skin absorption equation). (b) Estimate how it takes after exposure begins for the groundskeeper's plasma dieldrin levels to reach 1 ng/L. (c) Estimate the maximum plasma dieldrin concentration in ng/L that will occur in this groundskeeper due to this exposure. (d) Estimate how long it will take after exposure ends for the groundskeeper's plasma dieldrin levels to drop back down to 1 ng/L. %3Darrow_forwardCl₂(g) + 2e Cr₂0 (aq) + 14 H(aq) + 6e¯ 02(g) +4H(aq) + 4e¯ MnO2(s) + 4H(aq) + 2e 103 (aq) +6H(aq) + 5e¯ VO₂ (aq) + 2H(aq) + e¯ Br₂() + 2e NO, (aq) + 4H(aq) + 3e¯ ClO2(g) + e Ag+(aq) + e 2 CT (aq) 1.36 2 Cr3+(aq) + 7 H₂O(1) 1.33 2 H₂O(1) 1.23 Mn2+(aq) + 2 H2O() 1.21 12(aq) + 3H2O(l) VO2+(aq) + H2O(l) 1.20 1.00 2 Br (aq) 1.09 NO(s) + 2 H2O(l) CIO₂(aq) 0.96 0.95 Ag(s) 0.80 0.77 Fe3+(aq) + e Fe2+(aq) A mixture of Fe3+, CIO₂, and Cr₂O,² was made, what reaction would be predicted, if any? a. Fe3+ + Cr2O7²- b. Cr2O + Clo²- c. Fe3+ Clo²- d. No reaction. -> -> Fe²+ + Cr³+. - Cr3+ + ClO2. Fe²+ + ClO2.arrow_forward
- 1-arr In a study of the gas phase decomposition of phosphine at 120 °C PH3(g)- →1/4 P4(g) + 3/2 H2(g) the concentration of PH3 was followed as a function of time. It was found that a graph of In[PH3] versus time in seconds gave a straight line with a slope of -2.50×10² s1 and a y-intercept of -3.24. Based on this plot, the reaction i v O order in PH, and the half life for the reaction is seconds. zero first secondarrow_forwardFor a field ecology project, you count the number of individuals of different ages found in a population during a single time period. There are 100 newborns, 40 1-year-olds, 15 2-year-olds, 5 3-year-olds, and 0 4-year-olds. Use these data to fill in the Nx, Sx, and Ix columns of a static life table.arrow_forwardNeed help with homeworkarrow_forward
- 1 1/2 yr to months using dimensional analysisarrow_forward5. This question has two parts a. and b. a. Assuming 100% yield, how much hexynyl lithium (in mmol) is produced from the reaction of LiHMDS with 1- hexyne? (mmol) b. Insert an image (.jpeg, png, .heic) showing all of your calculations for the moles of hexynyl lithium above. Note: Click on the image icon in the box below to upload/attach an image file. Warrow_forwardO O P AIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY