
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
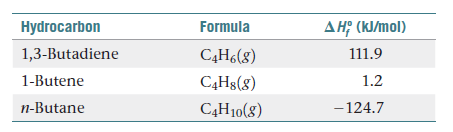
Three common hydrocarbons that contain four carbons
are listed here, along with their standard enthalpies of
formation:
(a) For each of these substances, calculate the molar enthalpy
of combustion to CO2(g) and H2O(l). (b) Calculate
the fuel value, in kJ/g, for each of these compounds. (c) For
each hydrocarbon, determine the percentage of hydrogen
by mass. (d) By comparing your answers for parts (b) and (c),
propose a relationship between hydrogen content and fuel
value in hydrocarbons.
Transcribed Image Text:дн (Ктol)
111.9
Hydrocarbon
Formula
1,3-Butadiene
1-Butene
C̟H6(g)
CĄHg(8)
C4H10(8)
1.2
n-Butane
-124.7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 148 g sample of an unknown metal at 74.0 oC is placed in a beaker containing 80.0 g of H2O at 25 oC. The final temperature of the metal sample and water is 32 oC. cH2O(l) = 4.184 j/ g oC SHOW ALL WORK (a) What is the specific heat (c) of the metal? (b) 5 common metals have the following specific heat capacities (c) [given in units of J/g oC] : Aluminum: 0.921 Copper: 0.377 Gold: 0.126 Iron: 0.461 Nickel: 0.502 Your sample is most likely to be which metal?arrow_forwardAmmonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes". Using the given data, calculate the heat of reaction for the following reaction: (NH4)½Cr207(s) –→ Cr2O3(s) + N½(g) +4H,O(g) AH (k mol) AG U mol K) ... Species mol") Nitrogem Ng) 191.5 Ng) NH,(g) 472.704 153.19 455.579 -46.11 192.3 -16.5 NH,(aq) NH,"(aq) - 80.29 III.3 -26.50 -132.51 113.4 -79.31 N,HC) 50.63 121.2 149.2 (NH),AsO/aq) -1268 NH,Br(s) -270.83 113 -175.2 NH,C6) NH,Caq) NH,HCO,6) -314.4 94.6 -201.5 -300.2 -847 12.1 -666 NH,I(s) -201.4 117 -113 NH,NO,) -3656 151.1 -184.0 (NH),SO) -1180.85 220.1 -901.67 NF(g) NO(g) NOg) -125 260.6 -83.3 90.25 210.7 86.57 33.2 240.0 31.30 N,Og) 82.05 219.7 104.2 N,O,(g) 83.72 321.28 139.46 N,Odg) 9.16 304.2 97.82 N,O(g) 356 115 -43.1 178 114 NOCIG) 52.59 264 66.36 HNO(0 -174.1 155.6 -80.79 HNO(g) -135.1 266.2 -74.77 HNO, (aq) -206.6 146 -110.5 Hydrogen H(g) 218.0 114.6 203.3 H(g) H,O() 130.6 -285.8 69.91 -237.2 -241.8 188.7…arrow_forwardWrite a balanced equation and draw an approximate enthalpy diagram for each of the following changes: (a) the sublimationof dry ice [conversion of CO₂(s) directly to CO₂(g)]; (b) the re-action of 1 mol of sulfur dioxide with oxygenarrow_forward
- Given the standard enthalpy changes for the following two reactions: ΔΗ° (1) N₂(g) + 2O2 (g) → 2NO2 (g) (2) 2N₂O(g) → 2N2(g) + O2(g) ΔΗ° = 66.4 kJ Standard enthalpy change : = - 164.2 kJ What is the standard enthalpy change for the following reaction? (3) 2N₂O(g) + 3O2 (g) → 4NO2 (g) AH° =? kJarrow_forward2. In studying the energy generated by a person, you may view the person roughly as a constant pressur calorimeter using sugar (i.e., sucrose, C₁2H₂2O11) to generate power. The reaction of table sugar in calorimeter is given by + Ch. 9 Sl....pdf C12H22O11(s)+120₂(g) →12CO₂(g)+11H₂O+heat. C. a. What is the heat released by consuming one mole of sugar (C₁2H22O11) in this b. Calculate the enthalpy of combustion per sugar utilization. mole Does the result of your calculation make I wi (540 Karak 14 11 M de 2009 1 AP fik Tour of 1 way? 20arrow_forward(a) John performed a bomb calorimetry experiment using 2.147 g of CSH10O5. The temperature of the calorimeter rose from 24.18°C to 28.33°C. Given the heat capacity of the calorimeter and contents was 4.567 kJ/°C, determine the heat of combustion of C3H10O5 in kilojoule per mole.arrow_forward
- ator%=Dassignment-take Not syncing The combustion of 0.1557 g benzoic acid increases the temperature of a bomb calorimeter by 2.50°C. Calculate the heat capacity of this calorimeter. (The energy released by combustion of benzoic acid is 26.42 kJ/g.) [References) Heat capacity = kJ/°C A 0.2155-g sample of vanillin (Cg Hg O3) is then burned in the same calorimeter, and the temperature increases by 3.20°C. What is the energy of combustion per gram of vanillin? Energy = kJ/g Per mole of vanillin? Energy = kJ/mol Submit Answer Try Another Version 5 item attempts remaining (Previous Next Save and Exit Email Instructor Technical Supportarrow_forward04) (g Calculate the volume of air at 30°C and 1.00 atm that is needed to burn completely 10.0 grams of propane. Assume that air is 21.0 percent O₂ by volume. The heat of combustion of propane is -2,220.1 kJ/molrxn. Calculate the heat of formati of propane given that AHO of H₂0(1) = -285.3 kJ/mol and AHO of CO2(g) = -393.5 kJ/ f AH-[(3•Co₂) + (4•H₂0)]-[C3H8 + 5(0₂)] [ F)+(4(-285.3)]-[AH C3H5] [200arrow_forwardConsider the reaction of ammonia gas with O2(g) according to the thermochemical equation given below: 4NH3(g) + 7O2(g) 4NO2(g) + 6H2O(1) AH° = -1132 kJ/mole (c) Write the thermochemical equation representing the combustion of 1 mole of ammonia. (d) Write the thermochemical equation representing the reaction of 1 mole NO2(g) with Hz0arrow_forward
- 5.67 (a) What is meant by the term standard conditions with zec erence to enthalpy changes? (b) What is meant by the ferm enthalpy of formation? (c) What is meant by the term standard enthalpy of formation? os gniwollol ori 19bian T0arrow_forwardPlease answer the question in the image with details.arrow_forwarddo the highlighted questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY