
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
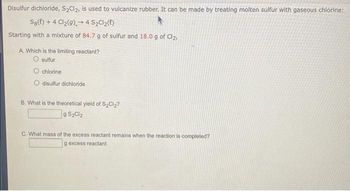
Transcribed Image Text:Disulfur dichloride, S₂Cl₂, is used to vulcanize rubber. It can be made by treating molten sulfur with gaseous chlorine:
Sg(t) + 4 Cl₂(g) 4 S₂Cl₂(l)
-
Starting with a mixture of 84.7 g of sulfur and 18.0 g of Cl₂,
A. Which is the limiting reactant?
O sulfur
chlorine
O disulfur dichloride
B. What is the theoretical yield of S₂Cl₂?
9 S₂Cl₂
C. What mass of the excess reactant remains when the reaction is completed?
g excess reactant-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. Calculate the percent yield for the reaction below if 75.0 g of phosphorous reacts with 345 g of chlorine to produce 111.0 g of phosphorous trichloride. P:(s) + 6 Cl,(g) –→ 4 PCI3(1)arrow_forwardThe combustion of propane occurs through the following chemical reaction. C3 H3 (g) + 5 O2 (g). - 3 CO2 (g) + 4 H,O (g) -> How many grams of H20 can be made by reacting 11.27 grams of oxygen gas?arrow_forwardNitric oxide (NO) reacts with oxygen gas to form nitrogen dioxide (NO2), a dark-brown gas: 2NO(g) + O (g) ⟶ 2NO (g) In one experiment 0.886 mole of NO is mixed with 0.503 mole of O2. Calculate which of the two reactants is the limiting reactant. Calculate also the number of moles of NO2 produced.arrow_forward
- Elemental silicon can be produced by the reduction of silicon dioxide, or sand, with carbon: SiO2 + 2 C ¡ Si + 2 CO When 35.0 kg of SiO2 reacts with 25.3 kg of carbon, and 14.4 kg of silicon is recovered, what is the percent yield for the reaction?arrow_forwardFill in the blanks using the other image.arrow_forwardA compound is composed of the elements, carbon, hydrogen, and oxygen only. Combustion of the compound produces CO2 and H2OC?H?O? + O2 ➝ CO2 + H2OCombustion of a 10.000 g sample of the compound produces the products shown in the table below. Product Mass (grams) CO2 22.837 H2O 5.843 Determine the empirical formula for the compound. [If an element has a subscript of 1, enter 1]Empirical Formula: CAnswerHAnswerOAnswerarrow_forward
- The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step process. In the first step, manganese(II) carbonate and oxygen react to form manganese(IV) oxide and carbon dioxide: 2MnCO,(G) + 0,(g) 2M1O,(G) + 2C0, (g) In the second step, manganese(IV) oxide and aluminum react to form manganese and aluminum oxidide: 3 MnO, (s) + 4Al(s) 3 Mn(s) + 2 Al,O3 (s) Suppose the yield of the first step is 95.% and the yield of the second step is 84.%. Calculate the mass of manganese(II) carbonate required to make 10.0 kg of manganese. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits. oloarrow_forwardWhen propane (C3Hg) reacts with oxygen, carbon dioxide and water are produced. The balanced equation for this reaction is C3 Hs (g) + 502 (g) → 3CO2(g) + 4H2O(g) If 10 moles of oxygen react, The reaction consumes moles of propane (C3H8). The reaction produces moles of carbon dioxide and moles of water.arrow_forwardWhen sulfuric acid reacts with calcium hydroxide, calcium sulfate andwater are produced. The balanced equation for this reaction is: H2S04(aq) + Ca(OH)2(s) CaSO4(s) + 2H2O(l) If 2 moles of calcium hydroxide react, The reaction consumes moles of sulfuric acid. The reaction produces moles of calcium sulfate and moles of water.arrow_forward
- A student reacts benzene, C6H6, with bromine, Br2, to prepare bromobenzene, CgH5Br, and HBr. C6H6 + Br2 --> C6H5Br + HBr What is the theoretical yield of bromobenzene in this reaction when 29.2 g of benzene reacts with 70.5 g of bromine? grams Which chemical is the limiting reactant? Select an answer If the actual yield of bromobenzene was 39.5 g, what was the percent yield?arrow_forwardCombustion of methane is an important fuel source. The combustion produces CO2 and H2O. What is the percent atom economy for the production of H2O in the reaction below?CH4(g) + 2O2(g) --> CO2(g) + 2H2O(g)arrow_forwardFor the following reaction, 15.9 grams of glucose (C6H1206) are allowed to react with 12.4 grams of oxygen gas > carbon dioxide(g) + water( glucose (CH1206(s) + oxygen(g) What is the maximum amount of carbon dioxide that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? gramsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY