
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Moving from left to right, do the dispersion forces get stronger, get
weaker, or stay roughly the same in the molecules shown here?
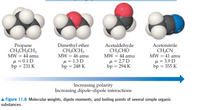
Transcribed Image Text:Propane
CH,CH,CH3
Dimethyl ether
CH,ÓCH,
MW = 46 amu
Acetaldehyde
CH,CHO
MW = 44 amu
u = 2.7 D
bp = 294 K
Acetonitrile
CH;CN
MW = 41 amu
MW = 44 amu
u = 0.1 D
bp = 231 K
µ = 1.3 D
bp = 248 K
µ = 3.9 D
bp = 355 K
Increasing polarity
Increasing dipole-dipole interactions
A Figure 11.8 Molecular weights, dipole moments, and boiling points of several simple organic
substances.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following organic reaction identify the mystery reactant R, its stoichiometric coefficient N, and any other substance X that must be present. CH3 CH₂ =C-C-CH=CH₂ || CH₂ Chemical formula of R: Stoichiometric coefficient N: + NR Must any other substance X be present? X CH3 | CH3 CH–CH–CH2–CH3 T CH3 If you said an additional substance X must be present, enter its chemical symbol or formula: 0 yes no 90 X 5arrow_forwardFor part B, it is not 4.98 x 10-41. I put it in and it was wrong. It is looking for P1.arrow_forwardThe spectroscopic data in the table is generated with five solutions of known concentration. Concentration (M) 0.0133 m= 0.0266 0.0532 0.106 0.213 Absorbance 0.1271 What is the intercept of the linear regression line? 0.08531 0.5388 1.069 Use a spreadsheet program, such as Microsoft Excel, to graph the data points and determine the equation of the best-fit line. 1.954 What is the slope of the linear regression line formed by these points? M-1arrow_forward
- After determining the absorbance of several standards of known concentration, the trendline for a calibration curve (AKA a standard curve) plotting absorbance (y-axis) against concentration (M, x-axis) is determined to be y = 4.943x + -0.0001 The absorbance of a solution of unknown concentration is determined to be 0.48. Calculate the concentration of the unknown solution in M. Give your answer to three decimal places.arrow_forwardOceanic uptake of carbon dioxide is thus described:CO2 (g) + H2O ⇔ H2CO3, K = [H2CO3]/PCO2 = 3 x 10-2 M atm-1 H2CO3 ⇔ HCO3- + H+, K = [HCO3-][H+]/[H2CO3] = 9 x 10-7 moles/LHCO3- ⇔ CO32 - + H+, K = [CO32 -][H+]/[HCO3-] = 7 x 10-10 moles/L CaCO3 ⇔ Ca2+ + CO32- Kc = 4 x 10-9 M2 What is the full charge balance equation for this system?arrow_forwardOceanic uptake of carbon dioxide is thus described:CO2 (g) + H2O ⇔ H2CO3, K = [H2CO3]/PCO2 = 3 x 10-2 M atm-1 H2CO3 ⇔ HCO3- + H+, K = [HCO3-][H+]/[H2CO3] = 9 x 10-7 moles/LHCO3- ⇔ CO32 - + H+, K = [CO32 -][H+]/[HCO3-] = 7 x 10-10 moles/LCharge balance equation:[H+] = [OH-] + [HCO3-] + 2[CO32 ] If the CO2 concentration in the atmosphere is 300 ppm, what is the pH of the ocean?arrow_forward
- professor Scimemi has accepted you as a Master’s student and you are involved in a project that studies the cellular basis of neuropsychiatric diseases. For your electrophysiology recordings you have to make a recording solution containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, 22 glucose. You need 800 ml of it. How much KCl do you need to weigh out? Hint: we only care about KCl here. (MW KCl is 74.55 g/molarrow_forwardYou are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role in liver disease. In the laboratory the enzyme was shown to have a Km of 1.0 x 10-6 M and Vmax of 0.1 micromoles/min.mg measured at room temperature. You developed an uncompetitive inhibitor. In the presence of 5.0 x 10-5 M inhibitor, the apparent Vmax was determined to be 0.02 micromoles/min.mg. What is the Ki of the inhibitor?arrow_forwardAfter determining the absorbance of several standards of known concentration, the trend line for a calibration curve plotting absorbance (y-axis) against concentration (uM, x-axis) is determined to be y=4.244x+0.0000. The absorbance of a solution of unknown concentration is determined to be 0.604. Calculate the concentration of the unknown solution in microM. Give answer to 3 decimal placesarrow_forward
- You are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role in liver disease. In the laboratory the enzyme was shown to have a Km of 1.0 x 10-6 M and Vmax of 0.1 micromoles/min.mg measured at room temperature. You developed a competitive inhibitor. In the presence of 5.0 x 10-5 M inhibitor, the apparent Km of the enzyme was found to be 1.5 x 10-5 M. What is the Ki of the inhibitor?arrow_forwardThe proton NMR spectrum of a compound, C3H6Cl2, has a pentet at d 2.19 and a triplet at & 3.72 with a 1:2 integration ratio, respectively. Which compound below best matches the data? A) CH₂CH₂CHC1₂ B) CICH₂CH₂CH₂C1 C) CH3CHCH₂C1 Cl C1 D) CH3CCH3 C1arrow_forwardAssign these protonarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY