
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
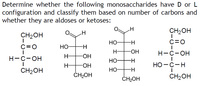
Transcribed Image Text:Determine whether the following monosaccharides have D or L
configuration and classify them based on number of carbons and
whether they are aldoses or ketoses:
CH2OH
CH2OH
НО-
C=0
C=0
НО-
H-
H-
HO-
Н-с-он
Н-с-он
H-
-HO-
НО
H-
H-
-HO-
НО -с-н
CH2OH
Но-
ČH2OH
CH2OH
CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which linear monosaccharide below corresponds to this cyclic one? A) A B) B C) C D) D E) None of themarrow_forwardExplain in detail the condensation reactions to form Maltose and Sucrose from their monosaccharides.arrow_forwardAll sugar residues are in their D-isomeric forms. Is this trisaccharide a reducing sugar? Why or why not?arrow_forward
- A)Label each sugar as D or L b. Label each sugar as a or Barrow_forwardThe polysaccharides are starch, glycogen, and cellulose. In the chart below, I put an 'X' under each polysaccharide that the statement applies to. I just wanted to verify my answers as I was confused for a few of them. Thanks! Glycogen Cellulose Starch Linear and minorly branched X X Complex carbohydrates X X X Insoluble X X X Unlimited storage in roots of plants X Fiber - digested by ruminants in living things X Beta glucose X For structure X Can't be digested X Extensive branching X Alpha glucose X X C, H, and O are backbone X X X Can be digested X X Limited storage in liver and muscle cells X Most abundant molecule X For energy X X Glycosidic links from condensation reactions X Cell walls X Found in Plants X X Found in Animals X Polymers of Glucose X X Xarrow_forwardAlthough the first two carbons of fructose and glucose are identical in structure to DHAP and GADP (from glycolysis), DHAP and GADP equilibriate on their in solution to favor the ketone over the aldehyde, while fructose and glucose do not. Why? a)The larger size of the molecule sterically hinders the isomerization b)The larger sugars have more OH groups which hydrogen bond and disrupt isomerization c)The larger sugars cyclize, and there is no carbonyl to isomerize in the cyclic form d)The larger sugars cyclize, and in the cyclic form the hydrogen bonding is very strong e)The larger sugars are less soluble in water than the smaller sugarsarrow_forward
- Which of the following statement regarding the ends of polysaccharides are true? for heads up 2 and 4 are not correct .. just pick only one answer 1) All polysaccharides have one, and only one, reducing end. 2) Some polysaccharides may have no reducing end. 3) Some polysaccharides may have no non-reducing ends. 4) All polysaccharides have a N-glycosidic bond at their reducing ends. 5) Some polysaccharides may have a functional group other than a carbonyl group at their reducing ends.arrow_forward18arrow_forwardExplain the structure and its componentsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON