
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
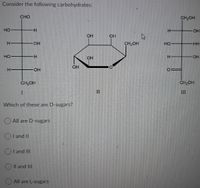
Transcribed Image Text:Consider the following carbohydrates:
CHO
CH2OH
но
H.
OH
OH
H.
OH
CH,OH
HO
HH
HO
OH
OH
OH
CH-OH
CH,OH
%3D
II
Which of these are D-sugars?
O All are D-sugars
I and II
I and IlI
Il and III
All are L-sugars
エ
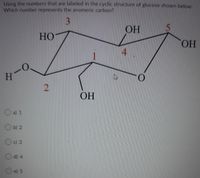
Transcribed Image Text:Using the numbers that are labeled in the cyclic structure of glucose shown below.
Which number represents the anomeric carbon?
3
OH
HO
HO.
4.
H
ОН
O a) 1
O b) 2
c) 3
d) 4
e) 5
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Jour CH,OH H. H. OH H но 3C OH H OH a-glucose CH,OH 0. OH OH H но 3C H. H OH B-glucose Compare the structure of two of these monosaccharides in a Venn dia- gram. Just like for other graphic organizers, the comparison should: •be meaningful; • be well-organized and easy-to-follow; • show your understanding of the vocabulary.arrow_forwardSelect all that applyarrow_forward6arrow_forward
- CH2OH CH2OH 12. What type of linkage is found in this carbohydrate? CH-O CH—0 Н A. a-1,4 В. В-1,4 CH OH CH CH С. а-1,6 D. B-1,6 ОН СН—СН CH-CH CH2OH ОН E. None of these is correct.arrow_forwardI choose all the wrong ones can i get the corrections to the right answers pleasearrow_forward15.arrow_forward
- Below is the structure of butyl alcohol. Based on the results of the experiment you just performed, predict the results of exposing beetroot cells to butyl alcohol. Explain your answer. H. H C-C-C- OH H H. H. Butyl Alcohol CIH HIC-arrow_forwardE Untit G pena G tylen G cyan b AnswM (no s Gmon EUX Sora Day Day ORBO6tBah5vDYVv95MSSaAqz8vjye5r9kgU/edit 目 Add-ons Help Last edit was 2 minutes ago ... BIUA 5田回 Arial 12 6. 4. 5 1 2 Observe the number of hydroxyl groups on cellulose, starch, and glycogen. What can the number of -OH bonds tell you about the molecule and its relationship with water? idsarrow_forwardI need answer expert solutions pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON