
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Determine the point group of the following molecules, identify the symmetry operations present,
and indicate if it is Polar or Nonpolar.
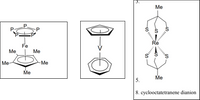
Transcribed Image Text:Ме
Fe
Me
Me
Me
Me
Ме
Ме
5.
8. cyclooctatetranene dianion
" .....
he
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List all the symmetry elements of a Cr(CO)6 molecule and identify the symmetry point group to which it belongs.arrow_forwardFind/show all possible symmetry operations and the point group of the following compounds by drawing their molecular structures. Additionally, decide whether the molecule is chiral or not. i) Decacarbonyldimanganese(0) ii) S4N4 iii) S2O8-2 iv) B2H6 v) Hydrazinearrow_forwardApply the symmetry operations of the point group to the B-Cl bond vector v1 to generate R(v1) for each symmetry operation of the group. Use the order and definition of the symmetry operations of the point group given in the table and the mutally perpendicular views of the B₂Cl4 molecule indicating the B-Cl bond vectors shown below to determine your answer. O v1 v4 v3 v2 v4 v3 v1 v2 O v1 v4 v3 v2 v3 v1 v4 v2 O v1 v3 v4 v2 v3 v4 v1 v2 O v1 v3 v2 v4 v3 v2 v1 v4 E clockwi santicloc kwise X SA se v4 CB- J=<+436 vl S4, C₂ y CH v3 B-B C₂ v2 C2 (1) C2 (2) od(xz) X Z C₂ (2) od(yz) Cl v3 v4 Cl-Cl C₂'(1) v2arrow_forward
- Give all the symmetry elements in the following molecules, and assign the point group of each. a ) Xe(0) F4 b) cyclo-(C5H5) - c) IF 7 (pentagonal bipyramidal) d) Boric acid (right - assume it's rigid)arrow_forwardPlease don't provide handwriting solutionarrow_forwardAutoSave O ff 331SYL22 - - O Search (Alt+Q) Yuleymi Gondola Protected View · Saved to this PC File Design References Mailings Review View Help P Comments v A Share Home Insert Draw Layout The following are some normal modes of vibration for several molecules. In each case: Determine the point group for the molecule Determine how the vibration transforms under each symmetry operation of the point and assign the appropriate irreducible representation State whether the vibration is IR active and/or Raman active Cote (benzene): C=C stretch c=C stretch a. e. "Breathing" CC twist C=C twist b. "Flexing" Asymmetric C-H and C-Ci stretches Asymmetric C-H and C-Ci stretches C. g. Symmetric C-H and C-Cl stretches Symmetric C-H and C-CI stretches Asymmetric C-H stretches d. h. k. Page 6 of 6 3011 words Text Predictions: On O Focus 100% 12:01 PM O Type here to search 12% 4 71°F * 4) ENG 3/23/2022 19 近arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY