
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Inbo
(534)
Conv
I Balar
b Ansv
Post
CHE
101 C X
с Chec
bartl
bartl
The
b My C
Unkr
O Sear
E I ma
G what
app.101edu.co/#
Bryant's Gmail
Cascadia Canvas Lo... T GSBA Scholarship L...
HOMEGROWN TRA...
Learn Touch Typing...
C The Science of Well...
Investor360° ® Login
ClickUp
Reading list
>>
Question 39 of 40
Submit
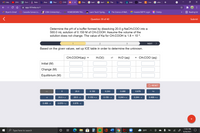
Determine the pH of a buffer formed by dissolving 20.0 g NaCH:COO into a
500.0 mL solution of 0.150 M of CH:COOH. Assume the volume of the
solution does not change. The value of Ka for CH:COOH is 1.8 x 10-5.
1
2
NEXT >
Based on the given values, set up ICE table in order to determine the unknown.
CH:COOH(аq) +
H2O(1)
H;O*(aq)
+ CH:COO-(aq)
Initial (M)
Change (M)
Equilibrium (M)
5 RESET
20.0
0.150
0.244
0.488
0.678
+x
20.0 + x
20.0 - x
0.150 + x
0.150 - x
0.244 + x
0.244 -
0.488 + x
-X
0.488 - x
0.678 + x
0.678 - x
+
11:03 PM
e Type here to search
59°F
W
8/26/2021
(8)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 1.0m HCl solution has a volume of 20.0mL. Calculate pH of the solution that results from 3.00g of solid acetate, NaCH3CO2, (82.04g/mol; Ka for CH3CO2H is 1.8x10^-5arrow_forwardq req ereg 2req 2req Use the References to access important values if needed for this question. A buffer solution contains 0.474 M NaH₂PO4 and 0.290 M K₂HPO4. If 0.0193 moles of sodium hydroxide are added to 125 mL of this buffer, what is the pH of the resulting solution? (Assume that the volume does not change upon adding sodium hydroxide) pH = Submit Answer Retry Entire Group 9 more group attempts remaining Previous Email Instructor Next Save and Exarrow_forwardA chemist titrates 130.0 mL of a 0.7778 M lidocaine (C,„H NONH) solution with 0.1602 M HCl solution at 25 °C. Calculate the pH at equivalence. The 14 pK, of lidocaine is 7.94. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added. dlo pH = | Explanation Check 2021 McGravw-Hill Education. All Rights Reserved Terms of Use PrivacyI Accessibility • 12:00 acer cspa 08. parrow_forward
- The pH of a bicarbonate-carbonic acid buffer is 5.63. Calculate the ratio of the concentration of carbonic acid (H₂CO3) to that of the bicarbonate ion (HCO3). Be sure your answer has the correct number of significant digits. Note: Reference the pK, of acids at 25 °C table for additional information. [HCO3] [H₂CO3]]arrow_forwardDetermine the pH of the resulting solution when the following two solutions are mixed: 50.0 mL of 0.500M HNO. and 30.0 mL of 0.200 M Ca(OH)2. The value of Ka for HNO: is 6.8 x 104. 1 2 3 4 NEXT > Use the table below to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of liquid water in the reaction. HNO2(aq) OH (aq) H2O(1) NO: (aq) + + Before (mol) Change (mol) After (mol)arrow_forwardA 17.8 mL solution of 0.100 mol L-1 NaOH is titrated using 0.150 mol L-1 HCl. What is the pH of the solution after 3.37 mL of the HCl solution is added? Express your answer to 2 decimal places.arrow_forward
- The p?a of hypochlorous acid is 7.530 . A 58.0 mL solution of 0.130 M sodium hypochlorite ( NaOCl ) is titrated with 0.299 M HCl . Calculate the pH of the solution after the addition of 9.78 mL of 0.299 M HCl . (please type answer not write by hend)arrow_forwardAscorbic acid C5H7O4CO2H (Ka =7.9x10^-5) also known as vitamin C is an essential nutrient for all mammals. In lab it can also be used to make buffers. Calculate the pH of a buffer solution which is made by mixing 0.250 moles of ascorbic acid and 0.200 moles of the conjugate base sodium ascorbate in a total volume of 0.750 liters.arrow_forwardTwenty millimeters of 0.50N HOAc is diluted with water to 100mL, and the resulting solution is titrated with 0.50N HOAc. Calculate the pH value (a) at the start, (b) when 0.8 ml of the NaOH has been added, (c) at the equivalence point (when 20mL of NaOH has been added, (d) when 30mL of the NaOH has been added. Ka=1.8x10^-5arrow_forward
- A chemist titrates 210.0 mL of a 0.3206M ammonia (NH3) solution with 0.3768 M HCl solution at 25 °C. Calculate the pH at equivalence. The pK, of ammonia is 4.75. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added. pH = 0 Xarrow_forwardIf a fifth solution (solution E) is made by mixing 10mL of soln C and 10mL of soln D, how will adding 10 mL of water effect the pHarrow_forwardDoes pH (H+/OH-balance) affect the movement of Polident molecules in solutions? Does Polident contain acidic or basic ingredients? How will the Polident ingredients interacts with the H+/OH- balance in the different pH solutions?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY