
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
determine the molecular structure based on the spectroscopic data in the picture
explain for each picture
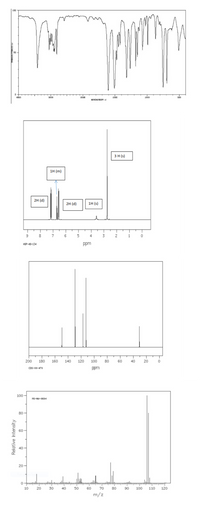
Transcribed Image Text:L00
50
3000
2000
1000
S00
KAVENUNE ERIl
3 H (s)
1н (m)
2H (d)
2H (d)
1H (s)
6.
8
7
6
4
2
1
HSP-49-134
ppm
200
180
160
140
120
100
80
60
40
20
ppm
CDS-00-470
100 -
HS-N-0684
80 -
60
40-
20 -
10
20
30
40
50
60
70
80
90
100
110
120
m/z
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
but at 6.60 ppm in the picture is 1 H (m/multiplet)
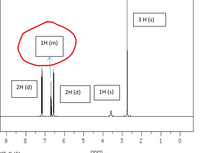
Transcribed Image Text:3H (s)
1н (m)
2H (d)
2H (d)
1H (s)
6
5
4
2
1
nnm
40174
3.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
but at 6.60 ppm in the picture is 1 H (m/multiplet)

Transcribed Image Text:3H (s)
1н (m)
2H (d)
2H (d)
1H (s)
6
5
4
2
1
nnm
40174
3.
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Structures of Molecular Compounds Formula Electron- Dot Structure Steric Number Electron Group Arrangement # of Bonded Atoms Molecular shape Polar? (Y/N) The questions are in the attached photo.arrow_forward8. For each molecular fomula, detem ine the electron pair geom etry (EPG), make a 3-dimensional representation, and determ ine the molecular geometry. Determine whether the molecule is polar or non- polar. All of these have central atoms surounded by term inal atom s/one pairs only. Formula A. CHF3 Lewis structure EPG 3-D drawing Mol Geom. Polar molecule? B. SF2arrow_forward1. Which one of the following combinations is incorrect? Group of answer choices CCl4, carbon (IV) chloride CrCl3, chromium (III) chloride CuO, copper (II) oxide FeO, iron (II) oxide 2. The strongest interactions between molecules of potassium oxide are dipole-dipole interactions hydrogen bonds dispersion forces ionic bonds nonpolar covalentarrow_forward
- Indicate the electron pair geometry and the molecular geometry for each of the six compounds.arrow_forwardFor each, draw the lewis structure, a 3D Structure including Angles, Electron Pair Geometry, Molecular Geometry, and whether it is "Polar or Non/Polar" A) SF6 B) CH2Oarrow_forwardPls helo ASAP. Pls help in all asked questions.arrow_forward
- Please note that "geometry" refers to the molecular or ionic geometry. H-N-H A. The Lewis diagram for NH3 is: The electron-pair geometry around the N atom in NH3 is There are lone pair(s) around the central atom, so the geometry of NH3 is B. The Lewis diagram for AsO2 is: Recall that for predicting geometry, double and triple bonds count as only one electron pair. The electron-pair geometry around the As atom in Aso2 is There are lone pair(s) around the central atom, so the geometry of AsO2 is Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Supportarrow_forwardWrite the Lewis dot STRUCTURE for EACH of the following compounds.You MUST show the process! Note: NO double bonds are in ANY of the compounds.a) XeF4b) SeCl3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY