
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
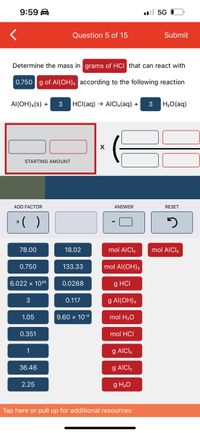
Transcribed Image Text:9:59 A
l 5G |
Question 5 of 15
Submit
Determine the mass in grams of HCI that can react with
0.750 g of Al(OH); according to the following reaction
Al(OH):(s) +
3
HCI(aq) → AICI3(aq) +
H2O(aq)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
78.00
18.02
mol AICI,
mol AICI,
0.750
133.33
mol Al(OH)3
6.022 x 1023
0.0288
g HCI
3
0.117
g Al(OH),
1.05
9.60 x 10-3
mol H20
0.351
mol HCI
1
g AICI3
36.46
g AICI3
2.25
g H20
Tap here or pull up for additional resources
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Lead(II) nitrate and ammonium iodide react to form lead(II) iodide and ammonium nitrate according to the reaction Pb(NO,),(aq) + 2 NH,I(aq) → PbI, (s) + 2 NH,NO,(aq) What volume of a 0.650 M NH4I solution is required to react with 889 mL of a 0.460 M Pb(NO3)2 solution? volume: mL How many moles of PbI2 are formed from this reaction? moles: mol PbI2arrow_forwardThe following chemical reaction takes place in aqueous solution: CuCl₂(aq)+Na₂S (aq) → CuS (s)+2 NaCl(aq) Write the net ionic equation for this reaction. 0arrow_forwardThe amount of I, (aq) in a solution can be determined by titration with a solution containing a known concentration of S₂O3(aq) (thiosulfate ion). The determination is based on the net ionic equation 2 S₂O3(aq) + ₂ (aq) → S₂O²(aq) + 31 (aq) Given that it requires 35.5 mL of 0.440 M Na₂S₂O3(aq) to titrate a 30.0 mL sample of I3 (aq), calculate the molarity of I3 (aq) in the solution. [1] = Marrow_forward
- Based on the balanced chemical equation shown below, determine the concentration (M) of Fe2+(aq) if 40.00 mL of the Fe2+(aq) solution is required to completely react with 43.47 mL of a 0.125 M bromate, BrO3-(aq), solution. The chemical equation for the reaction is 6 Fe2+(aq) + BrO3-(aq) + 6 H+(aq) → 6 Fe3+(aq) + Br-(aq) + 3 H2O(l) Do not type in units nor write in scientific notation.arrow_forwardIf 28.5 g of NaOH is added to 0.750 L of 1.00 M Cd(NO₃)₂, how many grams of Cd(OH)₂ will be formed in the following precipitation reaction? 2 NaOH(aq) + Cd(NO₃)₂(aq) → Cd(OH)₂ (s) + 2 NaNO₃(aq)arrow_forward3. What is the concentration of sodium chloride in the final solution if 28.24 mL of 0.129 M BaCl2 completely BaCl₂(aq) + Na₂SO4(aq) → reacts and the total volume of the reaction is 129.80 mL, given the reaction: BaSO4 (s) + 2NaCl (aq)arrow_forward
- The amount of chloride anion in a solution can be determined via titration with AgNO3(aq), according to the following balanced chemical equation: Cl–(aq) + AgNO3(aq) → AgCl(s) + NO3–(aq) What is the molarity of NaCl in a solution if titration of 15.00 mL of the solution with 0.2488 M AgNO3 requires 19.13 mL of the AgNO3 solution to reach the endpoint.arrow_forwardGive detailed Solutionarrow_forwardYou have 0.765 g of an unknown acid, H₂A, which reacts with NaOH according to the balanced equation H₂A(aq) + 2 NaOH(aq) → Na2 A(aq) + 2 H₂O(l) If 25.78 mL of 0.461 M NaOH is required to titrate the acid to the second equivalence point, what is the molar mass of the acid? Molar mass = g/molarrow_forward
- The reaction of hypothetical elements: A, B, C, D, and E, follows the molecular equation: AB2(aq) + CDE(aq) -> AE(s) + CB(aq) + DB(aq) Assuming that all aqueous compounds would fully dissociate, indicate the element/s that would form spectator ions in the subsequent total ionic equation.arrow_forwardGiven the reactant side of the total ionic equation for the neutralization reaction of sodium hydroxide (NaOHNaOH) with hydrochloric acid (HClHCl), write the total ionic equation (also known as the complete ionic equation) by entering both the reactant and the product species, separated by the reaction arrow. Na+(aq)+OH−(aq)+H+(aq)+Cl−(aq)→ ?Na+(aq)+OH−(aq)+H+(aq)+Cl−(aq)→ ? Be sure to include the charges on the ionic species and the physical state of all the reactant and product species. Express your answer as a chemical equation.arrow_forwardIf 31.0 g of NaOH is added to 0.550 L of 1.00 M Ni(NO₃)₂, how many grams of Ni(OH)₂ will be formed in the following precipitation reaction? 2 NaOH(aq) + Ni(NO₃)₂(aq) → Ni(OH)₂ (s) + 2 NaNO₃ (aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY