
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
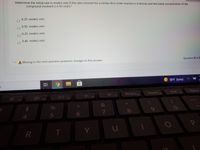
Transcribed Image Text:Determine the initial rate in mole/L·min if the rate constant for a certain first-order reaction is 0.40/min and the initial concentration of the
compound involved is 0.50 mol/L?
0.25 mole/L-min
0.50 mole/L·min
0.20 mole/L·min
0.40 mole/L·min
Question 5 of 2
A Moving to the next question prevents changes to this answer.
58°F Sunny
End
PgUp
Prt Scn F8
Home
F10
F9
F7
F6
F5
F4
F3
&
$4
7
4
5
Y
U
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1carrow_forwardA certain reaction is second order in N₂ and second order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. [N₂] [¹₂] initial rate of reaction 2.03 M 1.99 M 2.00 × 10 M/s 3 ? 2.03 M 0.942 M M/S 3.65 M 1.11 M M/Sarrow_forwardconsider the rate data at a certain temp for the reaction 2NO(g) + 2H2(g) --> N2(g) + 2H2O(g) determine the order of the reaction with respect to nitrogen monoxidearrow_forward
- Question in imagearrow_forwardThe stoichiometric equation for the reaction of nitric acid with oxygen is 2NO + O2 --> 2NO2. The following data was collected at 273°C. If the concentration of oxygen is doubled while holding the concentration of NO constant, the rate will increase by a factor of __. Experiment [NO] [O2] Initial Rate (M/s) 1 0.020 0.010 0.0320 2 0.080 0.020 1.01 3 0.040 0.040 0.50 4 0.040 0.020 0.25 5 0.030 0.010 0.71arrow_forwardA particular second order reaction has a rate constant of 0.0345 M-1 min-1 and an initial concentration of 0.300 M. What is the half-life of this reaction? 4.35 min 12.0 min 20.1 min 96.6 minarrow_forward
- This reaction is an example of conjugate addition of a nucleophile to an a,ß-unsaturated carbonyl. LOCH3 H20 H3C H3C° OCH3 OCH3 Draw the two resonance structures of the enolate anion intermediate for this reaction.arrow_forwardThe following set of data was obtained for the reaction below. What is the rate of reaction when [NO] = 0.00105M and [H2] = 0.0083M? . 2H2(8) + 2NO(8) 2H2O(g) + N2(8) Exp [NO], M [H2]. M Rate, M/s 1 0.0060 0.0010 0.180 2 0.0060 0.0020 0.360 3 0.0010 0.0060 0.030 4 0.0020 0.0060 0.120 5.0 x 106 Ms-1 43.5 Ms-1 -1 0.046 Ms 1.3 x 10-4 Ms-1arrow_forwardThe decomposition of O, is a second order reaction with a rate 3 constant of 50.5 M hr-¹. 2 O3(g) → 3 02 (9) What is the half-life for the decomposition of O, when the concentration of O, is 5.881×10¯ M? 1/2 Type numbers in the boxes. 10 points hrarrow_forward
- Part A Consider the balanced chemical equation. H2O2(aq) + 3I¯ (aq) + 2H+(aq) → I3 (aq) + 2H20(1) Predict the rate of change in the concentration of H2O2 (A[H2O2]/At). In the first 12.0 s of the reaction, the concentration of I drops from 1.000 M to 0.773 M . Express the rate to three significant figures and include the appropriate units. You may want to reference (Pages 587 - 592) Section 14.3 while completing this problem. ? A[H2O2]/At = Value Unitsarrow_forwardS A →>> B + C The 1st order reaction shown above has a rate constant of 2.00 x 103 min 1. If you start with [A] of 0.800 M, how long will it take for the [A] to drop to 0.600 M? O 207 min O 248 min O 144 min O none of the answers are correctarrow_forwardQuestion 7 of 8 Submit A reaction has a rate law of Rate = (1.25 M-1s-1)[A][B]. What is the rate of the reaction %3D if [A] = 0.301 M and [B] = 0.280 %3D %3D M? |M/s 1 4 5 6 C 7 8 9. +/- x 100arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY