
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
please very soon
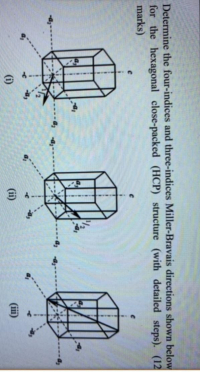
Transcribed Image Text:Determine the four-indices and three-indices Miller-Bravais directions shown below
for the hexagonal close-packed (HCP) structure (with detailed steps). (12
marks)
(i)
(ii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- True or false and why is false 1 There are 2 major classes of keratins: alpha keratins (found in mammals) and beta keratins (found in feathers and scales of birds and reptiles) 2 One estimate of mobility within a crystal is the B-factor that reflects spreading or blurring of electron density 3 In NMR, the longitudinal relaxation time (T1) reflects the rate at which magnetization returns to the longitudinal axis after a pulsearrow_forwardLogin AM McGraw Hill IT Central Auth MOTION EYE OWLv2 | Assic Content Which compound will be more likely to exist as a hydrated solid? Co(NO3)3 NaBr https://east.cengagenow.com/in/takeAssignment/takeCovalentActivity.do?locator=assignment-take OWLv2 | On [References]arrow_forward3. Transition metals of the periodic table are known to adopt either a tetrahedral or square planar configuration when it has a coordination number of 4. Consider the inorganic complex ZrI4. a) Draw the structure, determine all symmetry elements and operations, and assign point group classification of Zr14 if it is tetrahedral, and square planar. (10 pts) b) If Zrl is tetrahedral, how many IR absorption peaks is expected given its point group classification? (2 pts) c) If Zrl4 is square planar, how many IR absorption peaks is expected given its point group (2 pts) classification? d) The IR spectrum of gaseous Zrl4 shows absorption peaks at 55 cm-1 and 245 cm-1. This data is consistent with what shape of ZrI4. (2 pts)arrow_forward
- Which is a weaker acceptor (analogous to weaker acid)? In, CuWhich is a weaker donor (analogous to weaker base)? As, Mnarrow_forwardThe questions are on the picturesarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!arrow_forward
- 7. (10 points) Indicate the type of crystal (molecular, metallic, ionic, or covalent-network) each of the following would form upon solidification a. Na2CO3 molecular metallic ionic covalent-network b. Cu molecular metallic ionic covalent-network C. acetone molecular metallic ionic covalent-network d. Mg molecular metallic ionic covalent-network 5004 e. C molecular metallic ionic covalent-network 49arrow_forwardQuestion 47 of 80 Submit What is the correct IUPAC name for W(Cr,O,),? 2 (1) (11)) (III) ) (IV) heха-hepta-di- tetra- octa- tri- penta- mono- tungsten perchromate dichromate chromium oxygen chromide chromite chromate oxide + hydrate acid Deletearrow_forwardIf a Schottky defect concentration of 6.2 x 1010 /cm3 is observed in Al2O3, what is the total vacancy concentration (in 1/cm3) present in this sample of Al2O3? a. 2.3 x 108b. 3.1 x 1010c. 6.2 x 1010d. 1.24 x 1011e. 3.1 x 1011 When compared to that of a covalent material, the short-range atomic structure of amaterial assembled using ionic bonding can be characterized as having a:a) higher coordination numberb) lower atomic packing factorc) higher atomic packing factord) lower coordinationnumbere) a and c onlyarrow_forward
- New Tab + O 6 https://east.cengagenow.com iln/takeAssignment/takeCovalentActivity.do?locator=assignment-take O Other Bookmarks [References) Use the References to access important values if needed for this question. In the illustration, N is color-coded as blue and O is color-coded as red, What kind of change is represented by this illustration? Is energy absorbed or released when this change occuri sico vaporization Submit Answer Try Another ersion 2 item attemp sublimation freezing condensation deposition Provinus Next 6) 3:27 PM 5/5/2022 SONY DISPLA WEB One Touch Web Access Without Booting Up F12 Num Lk Ser Lk PrtSc Sys Rg Delete Break Insert F5 F6 F7 F8 F9 F10 F11 Pause 156 Backspace Home -- Page Up Enter K Page Down Enterarrow_forwardPlease answer Compare Column 1 with Column 2arrow_forwardOneLogin ALEKS - Kayley Valentine b nickel (II) sulfate cation and ani x Lea x + A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBI5ahvJxLWwcNXdqGDInlqlcnbb-P21X0OTA0ITA3YJ_7HPrxSXe3aRFsU.. Apps O R Prechecks: Test 2... O ATOMS, IONS AND MOLECULES Deducing the ions in a polyatomic ionic compound from its empir... Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation anion NaCl CI Na NiSO, V Cl, Cro, Fe (OH), Explanation Check O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use l Pr MaćBook Pro esc 888 F4 F1 F5 F6 F7 F8 F9 F10 F11 F12 24 & Q т H. alt olt tion command command optic * 00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning