
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
5. please dont submit AI. answer as its wrong
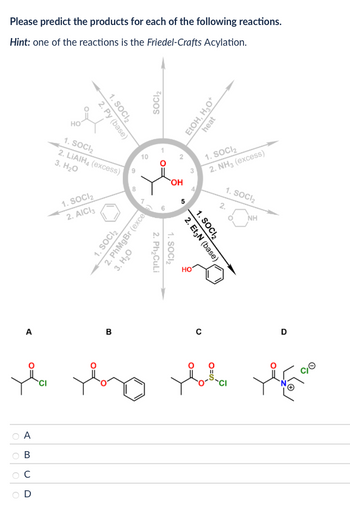
Transcribed Image Text:Please predict the products for each of the following reactions.
Hint: one of the reactions is the Friedel-Crafts Acylation.
A
ia
CI
A
B
C
D
1. SOCI₂
2. Py (base)
HO
1. SOCI₂
2. LiAlH (excess)
3. H₂O
1. SOCI₂
2. AICI 3
6
8
10
10
1. SOCI₂
2. PhMgBr (exce
3. H₂O
SOCI₂
1. SOCI₂
2.
Ph₂CuLi
OH
10
EtOH, H₂O+
heat
1. SOCI₂
2. NH3 (excess)
1. SOCI₂
2.
NH
1. SOCI₂
2. Et3N (base)
HO
B
C
D
хого увива доба
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is the purpose of HCl in the iodination of vanillin. And what is the complete mechanism for this reaction, along with how do you synthesize the catalyst and electrophil I-Cl.arrow_forward7. Reaction Scheme. NH₂ NH2 or two differnet methods (no same steps/reagents) C5H12N2 1. xs Mel, xs K2CO3 2. Ag2O, H₂O 3. heat Br2, xs NaOH, xs H₂O OHC 1.03 2. DMS CHOarrow_forwardThe synthesis of an azo dye is shown below. Please answer the following questions. NH2 1) Na2CO3 2) NaNO2, HCI B N-Ñ A Š3H ŠO3 Step 1 Step 2 ŠO3 2 1 1. Why you need to add Na2CO3 in step 1? (multiple answers) Oa)To decrease the solubility of compound 1 - "salting out" ob) To deprotonate compound 1, increase solubility in water Oc) To increase the reaction yield Od)To slow down the reaction rate Oe)To neutralize the HCIarrow_forward
- VII. Chemical Reactions and Mechanisms: Use the space below for reactions and mechanisms. Он о NH, NH3 он о но CH3 MeOH CH3 ÕH Но. Diastereomeric HO reflux Salts NH2 ÕH Он О CH3 NH3 CH3 ÕH (+)-1-phenylethanamine (+)-tartaric acid Alkaline Hydrolysis of the Diastereomeric Salt: OH O Он О Na NH3 NH2 OH 14 M NaOH Na CH3 ÕH CH3 ОНarrow_forward26. Which sequence of reactions would be the best choice to carry out the step below? Но HO а. d. 1. MgBr CI . AICI3 2. Но b. е. 1. / CI . AIC13 CI, AICI3 2. Zn(Hg), HCl с. 1. Br,. FeBr3 2. MgBrarrow_forwardUse the information below to identify and explain the relationship between the basicity of the two nucleophiles and their nucleophilicitiesarrow_forward
- SN1 reactions are better performed in protic solvents. Explain why the researchers could not perform the reaction using 100% water as solvent instead of 50% aqueous acetone.arrow_forwardPlease provide mechanism and structures for intermediate A and intermediate Barrow_forward18 of 21 60. Select the compound that is not an intermediate in the following organic transformation. ОН НО НО НО ОН ОН ОН А 61. When treated with 0.01 M HCI, molecule _will be protonated to the greatest extent while molecule will be protonated to the least extent. NH2 II IV || O=N* а. 1, I b. III, I C. III, II d. III, IV 62. Identify the mechanistic step in a Claisen condensation with the largest equilibrium constant value. a. initial proton transfer by ethoxide to yield the enolate ion b. nucleophilic attack of the enolate ion on the ester electrophile c. reformation of the carbonyl to promote heterolysis of the alkoxide leaving group d. proton transfer of the alkoxide on the newly formed beta keto ester substratearrow_forward
- Use the information below to identify and explain the relationship between the basicity of the two nucleophiles and their nucleophilicitiesarrow_forwardPredict major products for the following reactions g. h. H2SO4 d. e. NaOH A 1. .CI 1. Li(tBu),AIH من 2. H₂O+ 1. LiAlH4 2. H₂O+ 1. DIBAL-H, -78 °C 2. H₂O* 1. LiAlH4 2. H₂O+ 1. LiAlH4 J. OH 2. H₂O*arrow_forwardPredict the product for the following reaction sequence. OH Na₂Cr₂O7/H₂SO4/H₂O A. B. SOCI₂ EN D. excess NH3 SOCI₂ 1. CH3CH₂MgBr 2. H3O+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning