
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:1
2-
3C₂²-
10:31
+
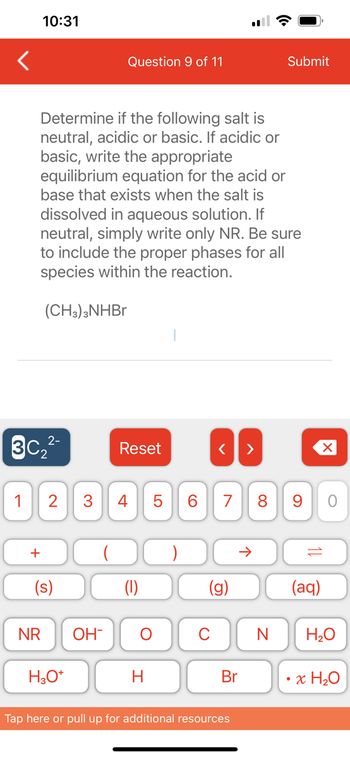
Determine if the following salt is
neutral, acidic or basic. If acidic or
basic, write the appropriate
equilibrium equation for the acid or
base that exists when the salt is
dissolved in aqueous solution. If
neutral, simply write only NR. Be sure
to include the proper phases for all
species within the reaction.
(CH3)3NHBr
NR
(s)
2 3 4
H3O+
Question 9 of 11
OH
Reset
O
H
LO
5
6
O
< >
7 8
Br
Tap here or pull up for additional resources
Submit
个
9
11
(aq)
O
N H₂O
x H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the indicators in Fig. 14-8 could be used for the titrations in Exercises 61 and 63?arrow_forward8-55 We commonly refer to a buffer as consisting of approximately equal molar amounts of a weak acid and its conjugate base—for example, CH3COOH and CH3COO-. Is it also possible to have a buffer consisting of approximately equal molar amounts of a weak base and its conjugate acid? Explain.arrow_forward. How is the strength of an acid related to the fact that a competition for protons exists in aqueous solution between water molecules and the anion of the acid?arrow_forward
- 8-112 Consider an initial 0.040 M hypobromous acid (HOBr) solution at a certain temperature. At equilibrium after partial dissociation, its pH is found to be 5.05. What is the acid ionization constant, Ka, for hypobromous acid at this temperature?arrow_forwardThe indicator dinitrophenol is an acid with a Ka of 1.1104. In a 1.0104 -M solution, it is colorless in acid and yellow in base. Calculate the pH range over which it goes from 10% ionized (colorless) to 90% ionized (yellow).arrow_forwardm-Nitrophenol, a weak acid, can be used as a pH indicator because it is yellow at pH above 8.6 and colorless at pH below 6.8. If the pH of a 0.010M solution of the compound is 3.44, calculates its pKa.arrow_forward
- For conjugate acidbase pairs, how are Ka and Kb related? Consider the reaction of acetic acid in water CH3CO2H(aq)+H2O(l)CH3CO2(aq)+H3O+(aq) where Ka = 1.8 105 a. Which two bases are competing for the proton? b. Which is the stronger base? c. In light of your answer to part b. why do we classify the acetate ion (CH3CO2) as a weak base? Use an appropriate reaction to justify your answer. In general, as base strength increases, conjugate acid strength decreases. Explain why the conjugate acid of the weak base NH3 is a weak acid. To summarize, the conjugate base of a weak acid is a weak base and the conjugate acid of a weak base is a weak acid (weak gives you weak). Assuming Ka for a monoprotic strong acid is 1 106, calculate Kb for the conjugate base of this strong acid. Why do conjugate bases of strong acids have no basic properties in water? List the conjugate bases of the six common strong acids. To tie it all together, some instructors have students think of Li+, K+, Rb+, Cs+, Ca2+, Sr2+, and Ba2+ as the conjugate acids of the strong bases LiOH, KOH. RbOH, CsOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2. Although not technically correct, the conjugate acid strength of these cations is similar to the conjugate base strength of the strong acids. That is, these cations have no acidic properties in water; similarly, the conjugate bases of strong acids have no basic properties (strong gives you worthless). Fill in the blanks with the correct response. The conjugate base of a weak acid is a_____base. The conjugate acid of a weak base is a_____acid. The conjugate base of a strong acid is a_____base. The conjugate acid of a strong base is a_____ acid. (Hint: Weak gives you weak and strong gives you worthless.)arrow_forwardIn dilute aqueous solution HF acts as a weak acid. However, pure liquid HF (boiling point = 19.5 C) is a strong acid. In liquid HF, HNO3 acts like a base and accepts protons. The acidity of liquid HF can be increased by adding one of several inorganic fluorides that ale Lewis acids and accept F- ion (for example, BF3 or SbF5]. Write balanced chemical equations for the reaction of pure HNO3 with pure HF and of pure HF with BF3.arrow_forwardDefine or illustrate the meaning of the following terms: a. amphoteric b. Kw reaction c. Kw equilibrium constant d. pH e. pOH f. pKw Give the conditions for a neutral aqueous solution at 25C, in terms of [H+], pH, and the relationship between [H+] and [OH]. Do the same for an acidic solution and for a basic solution. As a solution becomes more acidic, what happens to pH, pOH, [H+], and [OH]? As a solution becomes more basic, what happens to pH, pOH, [H+], and [OH]?arrow_forward
- 9:10 2- 3C₂²- + Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. C (CH3)2NH2CIO, 1 2 3 4 (s) Question 7 of 12 O CI Reset LO H₂O 5 1 6 7 8 6 H Submit 个 Tap here or pull up for additional resources 9 11 N OH- NR H3O+ (aq) O x H₂Oarrow_forward1 9:49 + C 2- 3C₂² Reset Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. CSC4H₂O6 2 3 Question 11 of 11 O OH 1 4 5 6 7 8 NR Cs H (g) Tap here or pull up for additional resources Submit H₂O (aq) • x H₂O × ( 0 H3O+arrow_forward4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning