
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
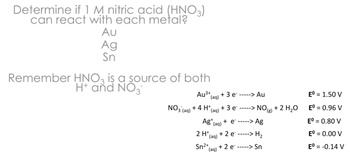
Transcribed Image Text:Determine if 1 M nitric acid (HNO3)
can react with each metal?
Au
Ag
Sn
Remember HNO3 is a source of both
H+ and NO3
NO3(aq)
Au³+ +3e
(aq)
+4 H+ +3 e
(aq)
Ag (aq)
+ e -----> Ag
2 H+ +2 e -----> H₂
(aq)
Sn2+(aq)
+2e -----> Sn
--> Au
Eº = 1.50 V
NO (8)
+2 H₂O
Eº = 0.96 V
Eº = 0.80 V
Eº = 0.00 V
Eº = -0.14 V
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Which below is the proper equation for dissociation of zinc hydroxide? a) Zn(OH)2 --> Zn + 2 OH b) ZnOH <--> Zn+ + OH- c) Zn(OH)2 <--> Zn + 2 OH d) ZnOH --> Zn + OH e) Zn(OH)2 --> ZnO + H2O f) ZnOH --> Zn+ + OH- g) Zn(OH)2 --> Zn+2 + 2 OH- h) Zn(OH)2 <--> Zn+2 + 2 OH-arrow_forwardWhat are the products of the complete reaction between sodium bicarbonate and aqueous sulfuric acid. Be sure to note states of matter and balance your equation. Group of answer choices a. NaHCO3(aq) + H2SO4(aq) --> H2O(l) + CO2(g) + Na2SO4(aq) b. NaHCO3(aq) + H2SO4(aq) --> H2CO4(g) + Na2SO4(aq) c. 2NaHCO3(aq) + H2SO4(aq) --> 2H2CO3(aq) + Na2SO4(aq) d. 2NaHCO3(aq) + H2SO4(aq) --> 2H2O(l) + 2CO2(g) + Na2SO4(aq)arrow_forwardThe Net Ionic Equation for the reaction of a solution of H2SO4 with a solution of Ba(OH)2 is __. a) H+ + OH- --> H2O b) 2H+ + 2OH- --> 2H2O c) H2SO4 + Ba(OH)2 --> BaSO4 + 2H2O d) 2H+ + SO42- + Ba2+ + 2OH- --> BaSO4 + 2H2O e) H2SO4 + Ba2+ + 2OH- --> BaSO4 + 2H2Oarrow_forward
- hi i'm struggling a bit balancing this equationarrow_forwardUse the following balanced chemical equation to answer the below question: 2H&PO4 (aq) +3Mg(OH)2 (aq) 6H2O m + Mg3(PO4)2 (s) The titration of a 14.0 mL sample of H3PO, requires 3.51 mL of 0.400 M Mg(OH)2 to reach the equivalence point. What is the concentration of H3PO,?arrow_forwardA solution contains S2– and SO42– ions. To separate each anion independently, add ___ first, then add ___. Question 19 options: Pb(NO3)(aq) , Ca(NO3)2(aq) Ag(NO3)(aq) , Ba(NO3)2(aq) Ba(NO3)2(aq) , Ag(NO3)(aq)arrow_forward
- Balance this equation in basic solution ClO2^- + S2O3^2- <-> Cl- + SO3^2-arrow_forwardThe balanced equation for the reaction between Co(OH)3 and K2S is: Group of answer choices a) 2 Co+3 + 3 K2S --> Co2S3 + 6 K+ b) 2 Co(OH)3 + 3 K2S --> 2 CoK3 + 2 S(OH)2 c) OH- + K+ --> KOH d) 2 Co(OH)3 + 3 K2S --> Co2S3 + 6 KOHarrow_forwardTOFF #0 11 D *Have you been balancing the chemical equation for each question? If not, go back and fix your work. 4. What volume, in L, of 0.150 M KOH is required to neutralize 20.00 mL of 0.1500 M H₂SO4? KOH(aq) + H₂SO4(aq) K₂SO4(aq) + H₂O(1) CA CRUarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY