
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:nt Score:
of 19
>
41.8%
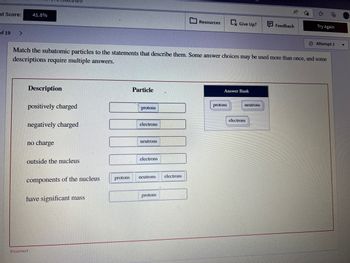
Description
positively charged
negatively charged
no charge
outside the nucleus
components of the nucleus
Match the subatomic particles to the statements that describe them. Some answer choices may be used more than once, and some
descriptions require multiple answers.
have significant mass
Incorrect
protons
Particle
protons
electrons
neutrons.
electrons
neutrons
protons
Resources
electrons
Ex Give Up?
Answer Bank
protons
neutrons
A
electrons
Feedback
to
Try Again
Attempt 1
Expert Solution
arrow_forward
Step 1
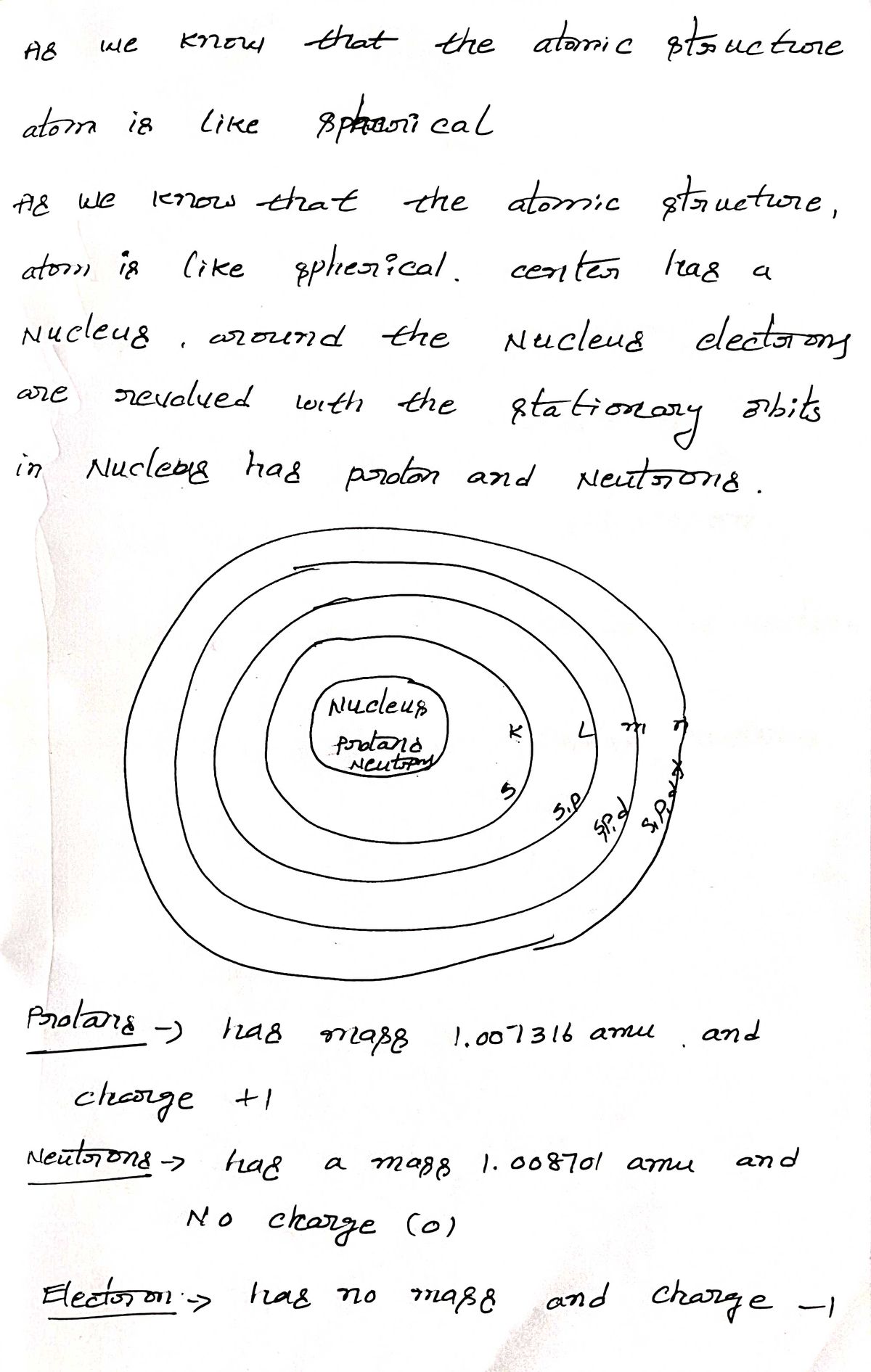
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which statement about the atom's nucleus is correct? O The nucleus is made of protons and neutrons and has a negative charge. The nucleus is made of protons and neutrons and has a positive charge. O The nucleus is made of electrons and has a positive charge. O The nucleus is made of electrons and has a negative charge.arrow_forwardWhat does the number 84 in the name Krypton-84 represent? the mass number None of these options are true the atomic number the sum of the protons and electrons O twice the number of protonsarrow_forwardFill in the table below by providing how many protons, and how many electrons in each atom/ion below. # of protons # of electrons # of neutrons in the most common isotope (just FYI) H 0 H+ 0 C 6 C+ 6 C- 6 O 8 O+ 8 O- 8 N 7 N+ 7 N- 7 S+ 16 P- 16 Cl 18 Br‑ 45arrow_forward
- The charge on an atom that has 15 protons and 18 electrons -3 -18 +15 +3arrow_forwardWrite the symbol for an atom/ion with the following information the ion with a 3+ charge, 28 electrons, and 40 neutronarrow_forwardAtoms of each element are unique in the composition of the atom. Atoms are determined to represent a particular element based on the number of within their nucleus. Group of answer choices Electrons Protons Nucleotides Neutronsarrow_forward
- Use this to fill in the following blanks: How many protons are in this atom? How many neutrons are in this atom? How many electrons are in this atomarrow_forwardWrite a isotope symbol for A cation with a charge of 3+, 41 electrons, 60 neutronsarrow_forward1.Which two subatomic particles each have a mass well below one atomic mass unit? Question options: all three of these subatomic particles have masses well below one atomic mass unit electons and protons neutrons and protons only one type of subatomic particle has a mass well below one atomic mass unit electrons and neutronsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY