
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
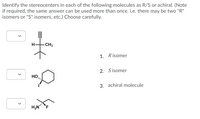
Transcribed Image Text:Identify the stereocenters in each of the following molecules as R/S or achiral. (Note
if required, the same answer can be used more than once. i.e. there may be two "R"
isomers or "S" isomers, etc.) Choose carefully.
H.
CH3
1. Risomer
2. Sisomer
HO
3. achiral molecule
>
Expert Solution
arrow_forward
Step 1
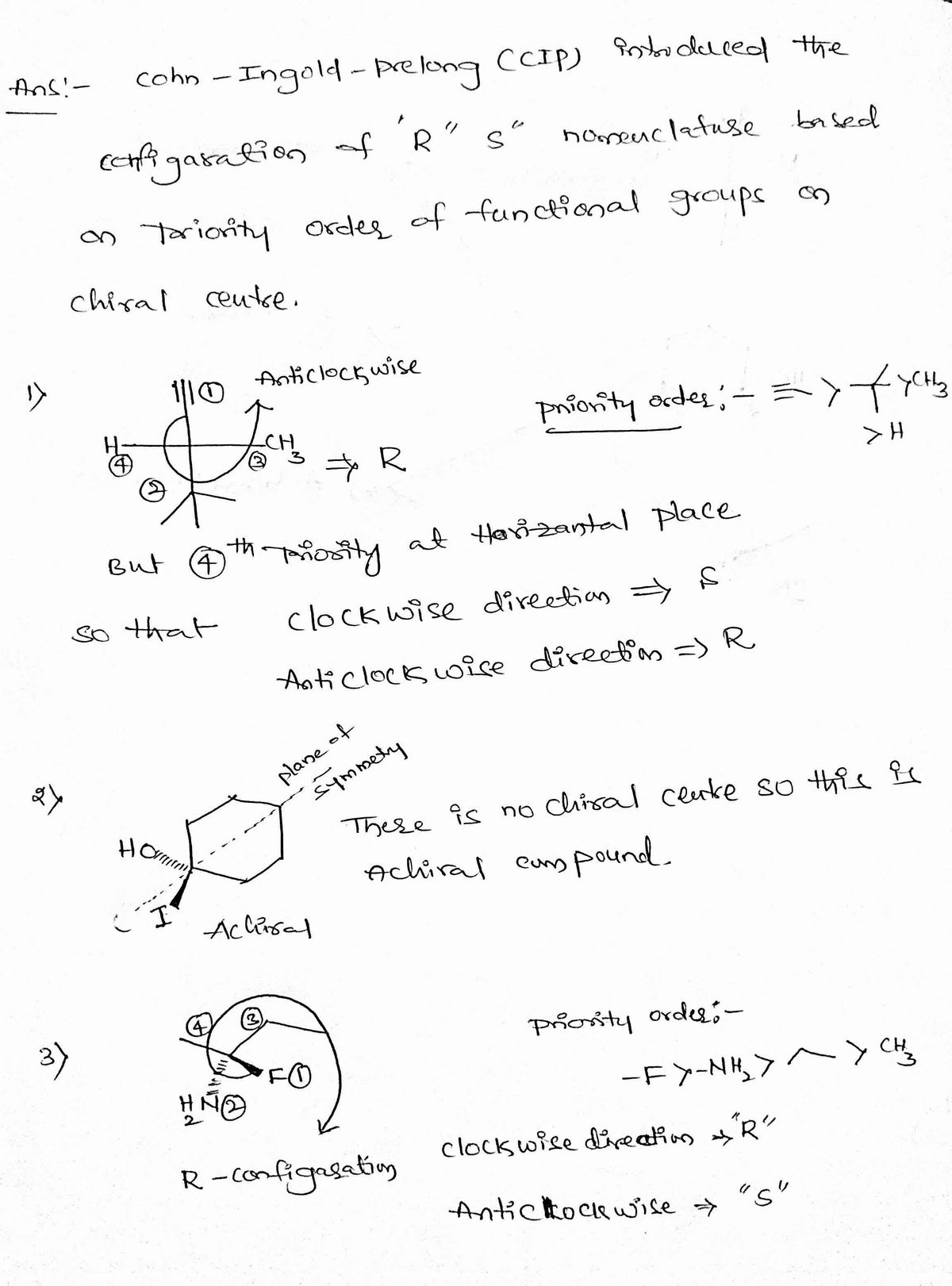
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Draw a structural formula of the SS configuration of the compound shown below. Mastered S S • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Include H atoms at chiral centers only. • If a group is achiral, do not use wedged or hashed bonds on it. Sn [F ? ChemDoodleⓇarrow_forwardare other unmodified S molecule from 4. Are they enantiomers or are they model with the identical? Draw the mirror image of this stereoisomer and assign the R/S configurations of each chiral carbons for both isomers. 6. Shown below is the Fisher projection of (R)-2-bromo-(S)-3-chlorobutane. CH3 -Br CH3 CI H. mirror plane 7. Make models of both stereoisomers in question 6. Test the models for superimposability. Secure the initials of your TA after showing them your physical model of (R)-2-bromo-(S)-3-chlorobutane. initials In the space below draw Fisher projections of the two stereoisomers of 2- thich are diastereomers of the stereoisomers shown in nnstructed for question 7 and switch two del represented by one of the mmetric centers.arrow_forwardPlease answer parts D,E,Farrow_forward
- Help pleasearrow_forward5. Instead of redrawing the molecule to make the lowest priority group point back, alternatively, the lowest priority group can be swapped with the group that is currently pointing back. This is now the enantiomer of the original compound. The procedure is described below. Determine priorities of the groups attached to the stereocenter. Swap the lowest priority group with the group that is pointing back, and redraw (this is the enantiomer). iii. Determine the absolute configuration (R or S) of the enantiomer. iv. Determine the absolute configuration (R or S) of the original compound. i. ii. Answer the following questions. Completely fill in the circle in front of your chosen answer. (a) Assign priorities of the groups at this stereocenter (1 = highest priority; 2 = second priority; 3 = third priority; 4 = lowest priority). Each group must have a different priority: CH3 Br H CH3 *Br H3CH₂C H 3 O 4 (b) The absolute configuration of this compound is: OR OS CH₂CH3 1 02arrow_forwardFor the following molecules: a. How many stereogenic centers are present? b. How many stereoisomers are possible for these molecules?arrow_forward
- For each organic compound in the table below, enter the locant of the highlighted side chain. compound CH 3 | CH₂ - CH - CH₂ - C - CH₂ 1 1 CH3 - CH₂ I CH3 CH3 CH₂ CH3 t CH₂-C- CH₂ - CH - CH₂ CH3 CH3 1 CH3 CH₂-C- | CH3 CH3 locant of highlighted side chain 0 3arrow_forwardDescribe the relationship of the following pairs of molecules as identical, enantiomers, diastereomers, or constitutional isomers. Br Ме, Н Me CI Me i. CI Br H Me H CI, Et Me CI Et Et. ii. Me Et Me CI Mee CI iii. none of the other answer choices are correct O i. = diastereomers; ii. = identical; iii. = enantiomers i. = diastereomers; ii. = constitutional isomers; iii. = diastereomers i. = enantiomers; ii. = constitutional isomers; iii. = diastereomers O i. = enantiomers; i. = identical; iii. = diastereomersarrow_forwardPlease help with parts K,L,N. If you notice any incorrect please let me know.arrow_forward
- For the molecule below, a) Draw out every possible stereosimer. b) Indicate any enantiomer pairs c) Then, indicate any meso-stereoisomers, and proceed to clearly indicate the internal mirror plane.arrow_forwardPlease answer parts G,H,Iarrow_forwardA)Circle all of the stereo centers in MDMA. B) assign the absolute stereochemistry (R or S) for each stereo centerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY