
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution .....
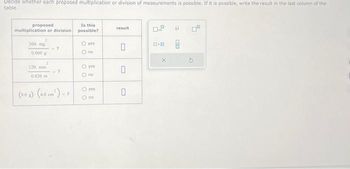
Transcribed Image Text:Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the
table.
proposed
multiplication or division
360 mg
0,000 g
2
120 mm
0.020 m
?
(9.0 g)-(4.0 cm²) -7
Is this
possible?
O yes
Ono
O yes
Ono
O yes
O no
result
0
0
0
ロ・ロ
H
ojo
d
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How would someone be confident in an experimentally determined concentration? Explain the reasoning using technical terms.arrow_forward[Revlew Topics] [References] Use the References to access important values if needed for this question. How many milliliters of 9.90 M hydrobromic acid solution should be used to prepare 4.50 L of 0.300 M HBr? mL Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardWhat experimental technique should you use to determine the concentration of a colored solution? O measurements of volume & mass calorimetry O spectrometry O titration aarrow_forward
- [References] Use the References to access important values if needed for this question. In the laboratory you dissolve 15.7 g of cobalt(II) fluoride in a volumetric flask and add water to a total volume of 125. mL. What is the molarity of the solution? Marrow_forward2) You are tasked with determining the barium content of a sample containing barium nitrate mixed with rubidium nitrate. The sample is weighed, dissolved into 50.0mg water, and treated with xs 0.500M sodium sulfate. The acid fully ionizes in this experiment. A white precipitate forms which is washed, filtered, and weighed multiple times, according to the data given below: Mass of sample 0.425g Mass of thoroughly dried filter paper 1.462g Mass of precipitate+filter after 1st drying 1.755g Mass of precipitate+filter after 2nd drying 1.699g Mass of precipitate+filter after 3rd drying 1.698garrow_forwardMasses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY