
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
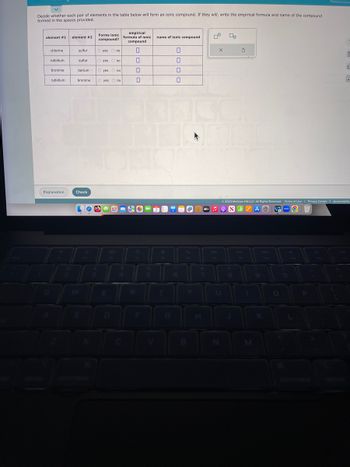
Transcribed Image Text:Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound
formed in the spaces provided.
element #1 element #2
chlorine
rubidium
bromine
rubidium.
Explanation
sulfur
sulfur
Forms ionic
compound?
O yes no
Check
O yes no
barium. O yes no
bromine O yes no
20
empirical
formula of ionic
compound
0
0
4
R
0
5
name of ionic compound
0
T
G
0
0
0
m
+
dtv ♫
8
X
00
Ś
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
C
1
(
M
9
7
O
d
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation + Na + Na + Na Na anion BrO 4 BrO₂ BrO₂ 3 Bro some ionic compounds empirical formula Ú 1 name of compound X Sarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Sn 2+ Ca 2+ Ba 3+ Mn anion 104 NO₂ CIO3 PO³ some ionic compounds empirical formula name of compound Xarrow_forward= O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Zn 4+ Pb 2+ Mg anion co CIO 3 So Some ionic compounds empirical formula 0 0 name of compound 0 X Sarrow_forward
- Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided.arrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation anion Fe2+ 103 c104 C102 Na Na Some ionic compounds empirical formula 0 name of Lompound X 00 Sarrow_forwardI'm having some trouble on my homework and could really use some help See attached:arrow_forward
- O ATOMS, IONS AND MOLECULES Naming binary ionic compounds Fill in the name and empirical formula of each ionic compound that could be fa cation anion + Cs + Cs + Home Cs + Cs Cl I Br F My Course - Introductory Biochemistry - Some ionic compounds empirical formula name of compoundarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Mg K anion F 0²- 1³. Cs² s² Some ionic compounds empirical formula 0 name of compound 0 Xarrow_forwardin the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Sn 3+ Fe NH + anion 103 C103 103 Some ionic compounds empirical formula 0 name of compoundarrow_forward
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 3+ Cr 2+ Mg 2+ Zn anion CH,CO, NO3 PO3 Some ionic compounds empirical formula name of compound Xarrow_forwardDecide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided. empirical formula of ionic name of ionic compound compound Forms ionic element #1 element #2 compound? cesium bromine O yes O no sodium sulfur O yes O no strontium potassium O yes O no chlorine magnesium O yes O noarrow_forward= O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions cation Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Na 2+ Zn 2+ Fe anion Shein Yahoo Search Results 103 CH,CO, C₂H₂02 S Search sports bra | SHEIN USA Some ionic compounds empirical formula name of compound X y raidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY