
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
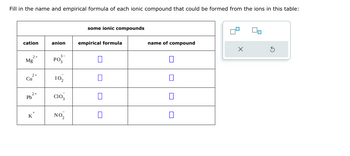
Transcribed Image Text:Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
cation
2+
Mg
2+
Cu
2+
Pb
K
anion
3-
PO³-
10₂
C103
NO₂
some ionic compounds
empirical formula
name of compound
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- = O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Zn 4+ Pb 2+ Mg anion co CIO 3 So Some ionic compounds empirical formula 0 0 name of compound 0 X Sarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound NH MnO4 ☐ ☐ 4 Sn 4+ 103 ☐ ☐ Mg 2+ NO, ☐ ☐ 5arrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound 2+ Fe 3+ Fe Cs Iarrow_forward
- Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided. empirical formula of ionic Forms ionic element #1 element #2 name of ionic compound compound? compound potassium barium O yes no cesium iodine yes no bromine potassium yes no sulfur chlorine yes noarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation anion Fe2+ 103 c104 C102 Na Na Some ionic compounds empirical formula 0 name of Lompound X 00 Sarrow_forwardFill in the name and empirical formula of each ionic compound that could b cation anion 2+ Sr 2+ Sr 2+ Sr 2+ Sr s²- 2- S ०२ N ³- 3- p³- P Some ionic compounds empirical formula name of compound 0 0arrow_forward
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Mg K anion F 0²- 1³. Cs² s² Some ionic compounds empirical formula 0 name of compound 0 Xarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds empirical formula name of compound cation anion 4+ Pb NO, 2+ Cu BrO4 2+ Mg co 2- CO3arrow_forward= O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions cation Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Na 2+ Zn 2+ Fe anion Shein Yahoo Search Results 103 CH,CO, C₂H₂02 S Search sports bra | SHEIN USA Some ionic compounds empirical formula name of compound X y raidearrow_forward
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds name of compound anion empirical formula cation 2+ Pb po Cr 3+ BrO3 2+ Mg NO,arrow_forwardNaming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ic cation K K 2+ Zn Explanation anion CIO CIO 3 CH,CO, Check Some ionic compounds empirical formula name of compoundarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Ba K Na 2+ Ca anion Bro₂ 2- so 102 PO some ionic compounds empirical formula name of compoundarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY