
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:f
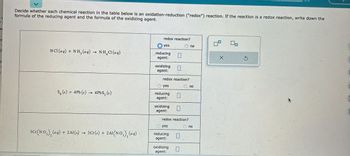
Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the
formula of the reducing agent and the formula of the oxidizing agent.
HCl(aq) + NH₂ (aq) → NH₂Cl(aq)
Sg(s) + 4Pb (s) → 4PbS₂ (s)
3Cr(NO₂)₂ (aq) + 2Al(s) 3 Cr(s) + 2A1(NO₂), (aq)
redox reaction?
yes
reducing
agent:
oxidizing 11
agent:
redox reaction?
yes
1
reducing 11
agent:
oxidizing
agent:
redox reaction?
yes
reducing
agent:
oxidizing
agent:
no
no
no
5
X
0
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the complete ionic equation for the reaction (if any) that occurs when aqueous solutions of H2SO4 and KOH are mixed. OH(aq) + OH¯(aq) → H₂O(l) O H+ (aq) + OH(aq) + 2 K+ (aq) + SO4²-(aq) → H+ (aq) + OH¯(aq) + K₂SO4(s) O No reaction occurs. O 2 K+ (aq) + SO4²- (aq) → K₂SO4(s) H+ (aq) + OH Taq) + 2 K+ (aq) + SO42 (aq) → H₂O(l) + 2K+ (aq) + SO4²- (aq)arrow_forwardWhat are the spectator ions in the following chemical reaction? Mg(NO3)2(aq) + 2 NaOH (aq) àMg(OH)2(s) + 2 NaNO3(aq)arrow_forwardThe traditional method of analysis for the amount of chloride ion present in a sample is to dissolve the sample in water and then slowly to add a solution of silver nitrate. Silver chloride is very insoluble in water, and by adding silver nitrate, it is possible effectively to remove all chloride ion from the sample. Ag+(aq) + Cl-(aq) → AgCl(s) Suppose a 1.515 g sample is known to contain 27.5% chloride ion by mass. What mass of silver nitrate must be used to completely precipitate the chloride ion from the sample?arrow_forward
- W Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. O 2 H* (aq) + CO3²-(aq) → H₂CO3(s) 2 Na* (aq) + CO3²- (aq) + 2 H*(aq) + 2 Cï(aq) → H₂CO3(s) + 2 NaCl(aq) O 2 H* (aq) + CO3²-(aq) → H₂O(1) + CO₂(g) 2 Na* (aq) + CO3²- (aq) + 2 H* (aq) + 2 Cl(aq) → H₂CO3(s) + 2 Na*(aq) + 2 Cl(aq) No reaction occurs.arrow_forwardWhat is the correct net ionic equation for the following double displacement reaction? Mg(NO3)2 (ag) +2 KF (ag) MgF (s) + 2 KNO, (ag) → a. Mg+2 (ag) + F (ag) M9F2 (s) b. Mg*2 (aq) +2 NO, (ag) + F (aq) → MgF, (s) +2 NO, (aq) c. Mg*2 (ag) + 2F (ag) - MgF2 (s) d. Mg*2 (ag) + (aq) - MgF, (s) c. K* (ag) + NO, (ag) KNO, (aq)arrow_forwardDetermine if the following reaction is a redox reaction. Use evidence from the equation to explain your reasoning.arrow_forward
- If 16.5 g of NaOH is added to 0.550 L of 1.00 M Ni(NO₃)₂, how many grams of Ni(OH)₂ will be formed in the following precipitation reaction?2 NaOH(aq) + Ni(NO₃)₂(aq) → Ni(OH)₂ (s) + 2 NaNO₃ (aq)arrow_forwardGive the complete ionic and net ionic equation for the reaction below. Circle the spectator ions. Fe(s) + 2 AgNO3(aq) → 2 Ag(s) + Fe(NO3)2(aq)arrow_forwardThe following chemical reaction takes place in aqueous solution: CuCl₂(aq)+Na₂S (aq) → CuS (s)+2 NaCl(aq) Write the net ionic equation for this reaction. 0arrow_forward
- To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.3600M silver nitrate AgNO3 solution to a 24.00g sample of the fluid and collects the solid silver chloride AgCl product. When no more AgCl is produced, he filters, washes and weighs it, and finds that 1.28g has been produced. The balanced chemical equation for the reaction is: Cl−(aq) + AgNO3(aq) -> AgCl(s) + NO−3(aq) What kind of reaction is this? If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Cl in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardIf 28.5 g of NaOH is added to 0.750 L of 1.00 M Cd(NO₃)₂, how many grams of Cd(OH)₂ will be formed in the following precipitation reaction? 2 NaOH(aq) + Cd(NO₃)₂(aq) → Cd(OH)₂ (s) + 2 NaNO₃(aq)arrow_forwardWhat is the balanced net ionic chemical equation between Cu^2+ (aq) and I^- (aq)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY