
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
feedback to help with question - Most of you managed this well once you realised that for Henry’s Law, the temperature should be approximated as the boiling temperature of the bulk component. This allowed the pure component vapour pressure of the dilute component to be evaluated. For the comparison with the x-y diagram, the determination of the initial gradient was approximate, but the value you found should certainly have corresponded more closely to the non-ideal Henry’s Law constant. This comment applies both to part (a) and part (b).
ans = a) (i) 3.48 atm; (ii) 7.21 atm, b) (i) 0.242 atm; (ii) 0.449 atm
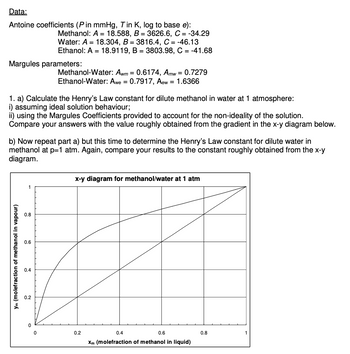
Transcribed Image Text:Data:
Antoine coefficients (P in mmHg, Tin K, log to base e):
Margules parameters:
1. a) Calculate the Henry's Law constant for dilute methanol in water at 1 atmosphere:
i) assuming ideal solution behaviour;
ii) using the Margules Coefficients provided to account for the non-ideality of the solution.
Compare your answers with the value roughly obtained from the gradient in the x-y diagram below.
Ym (molefraction of methanol in vapour)
b) Now repeat part a) but this time to determine the Henry's Law constant for dilute water in
methanol at p=1 atm. Again, compare your results to the constant roughly obtained from the x-y
diagram.
0.8
0.6
0.4
Methanol: A = 18.588, B = 3626.6, C = -34.29
Water: A = 18.304, B = 3816.4, C = -46.13
Ethanol: A = 18.9119, B = 3803.98, C = -41.68
0.2
0
Methanol-Water: Awm = 0.6174, Amw = 0.7279
Ethanol-Water: Awe = 0.7917, Aew = 1.6366
0
x-y diagram for methanol/water at 1 atm
0.2
0.4
Xm (molefraction of methanol in liquid)
0.6
0.8
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ESA.8(b) At 310 K, the partial vapour pressures of a substance B dissolved in a liquid A are as follows: 0.010 0.015 0.020 P/kPa 82.0 122.0 166.1 Show that the solution obeys Henry's law in this range of mole fractions, and calculate Henry's law constant at 310K.arrow_forwardO: 18.08arrow_forward15. 2 Plot the vapour pressure data for a mixture of benzene (B) and ethanoic acid (E) given below and plot the vapour pressure / composition curve for the mixture at 5 0 \ deg C. Then confirm that Raoult's and Henry's laws are obeyed in the appropriate regions. Deduce the activities and activity coefficients of the components on the Raoult's law basis and then, taking B as the solute, its activity and activity coefficient on a Henry's law basis. Finally, evaluate the excess Gibbs energy of the mixture over the composition range spanned by the data. 0 . 0 16 0 0.0439XE 0.08350.11380.17140.4840.9671.5351.892.45 Pi / kPa Pg / kPa 35.05 34.2933.2832.6430.900.36960.58340.29730.66040.84370.99 Xx3.834.845.363.316.767.29 Pp / kPa Pg / kPa 26.0820.4228.1618.0110.00.47arrow_forward
- Solid-liquid phase diagram of naphthalene-biphenyl system was formed by determination of arrest and break points in cooling curves of different composition mixtures as follows 82 283 2222 90 80 Temperature (°C) 111 70 1 50 40 30 20 10 0 Area Area 1 0.2 Phase Diagram Area Il Area IV Area III 0.8 0.4 0.6 Mole fraction of naphthalene 1 a. Write the phases of naphthalene and biphenyl in different areas on graph (assume that these molecules are completely miscible in liquid phase and immiscible in solid phase) of phases Explanations (Which phases present?) 12 b. Explain briefly the eutectic point and explain its position on grapharrow_forwardTwo components, A and B, were mixed forming a non-ideal solution and their liquid and vapor phases were allowed to equilibrate at 298 K and a pressure of 1 atm. The mole fraction of A in the liquid phase was found to be 0.380. The mole fraction of A in the vapor phase was 0.490. Calculate the activities and activity coefficients of both A and B in the solution on the basis of Raoult’s law. The vapor pressures of the pure liquids were P*(A) = 790 Torr and P*(B) = 600 Torr. Do these represent positive, negative, or no deviations from Raoult’s Law? Describe the intermolecular interactions that take place between A and B.arrow_forward2. Draw and label completely the liquid vapour phase diagram of the acetone chloroform system given the following data. a) Only one liquid phase exist b) There is an azeotrope at 64.0 mole% chloroform with taze = 64.4 °C. c) Pure acetone and chloroform boil at 56.1 and 61.2 °C respectively.arrow_forward
- Using the phase diagram for mixtures of cyclohexane and toluene, estimate: A) Boiling point of pure toluene _________ B) Boiling point (Tbp) of solution with molar fraction of toluene 30%.______ C) Molar fraction of toluene (in %) in the gas phase above the solution with molar fraction of toluene 30% at its boiling point Tbp. ____________ D) Approximate molar fraction of solvent which remains in the liquid phase upon heating of mixture with 60% of toluene to 100oC. _______________arrow_forwardA vapor mixture analyzes 60% heptane, 28% octane and 12% benzene by volume. At 50°C, the mixture is in equilibrium with a liquid solution containing the same components. Calculate: a) Total pressure of the vapor in kPa (4 decimal places) b) Mass composition of the liquid solution (4 decimal places) Use DIPPR constants and equation if necessary.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY