Question
please help with the following questions (part d and e only) based on the question in the picture
d. Find an expression for the velocity of the electron at each energy level. Calculate
the velocity of the electron at the lowest energy level.
e. Assume a hypothetical ion with Z protons, no neutrons and one electron. Above
which Z the velocity of the electron exceeds the speed of light? can such an atom
exist?
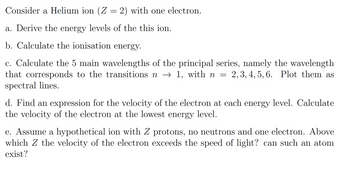
Transcribed Image Text:Consider a Helium ion (Z = 2) with one electron.
a. Derive the energy levels of the this ion.
b. Calculate the ionisation energy.
c. Calculate the 5 main wavelengths of the principal series, namely the wavelength
that corresponds to the transitions n → 1, with n = = 2, 3, 4, 5, 6. Plot them as
spectral lines.
d. Find an expression for the velocity of the electron at each energy level. Calculate
the velocity of the electron at the lowest energy level.
e. Assume a hypothetical ion with Z protons, no neutrons and one electron. Above
which Z the velocity of the electron exceeds the speed of light? can such an atom
exist?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Identify the unknown particles (A₂X) in the equations below. Show your work and mention the names of the particles. 1. 189F = 1780 + A₂X 2.4₂He + 147N = 1780 + A₂X 3.2₁H + 14₂N = 126C + A₂X 4, 238 892U = 234 90Th + + A₂X 5.³₁H=3₂He + A₂X + energy.arrow_forwardPlease I want a correct and clear solution to this question. The solution must be clear and handwritten. Course name: Lasers and its Applications 412PHYSarrow_forwardA rectangular corral of widths Lx = L and Ly = 2L contains seven electrons. What is the energy of (a) the first excited state, (b) the second excited state, and (c) the third excited state of the system? Assume that the electrons do not interact with one another, and do not neglect spin. State your answers in terms of the given variables, using hand me (electron mass) when needed.arrow_forward