Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
1. Give the major organic product(s) of the reaction and include all the stereochemistry as appropriate. Identify any meso compounds.
2. Indicate whether a solution of the product would be optically active or no not active and provide a simple reasoning to support your choice.

Transcribed Image Text:A.
B.
C.
D.
E.
1. Hg(OAc)2/H₂O
2. NaBH4
H₂
Pd/C
HBr
ROOR
Br₂
hv
H₂
Pd/C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
1. Give the major organic product(s) of the reaction and include all the stereochemistry as appropriate. Identify any meso compounds.
2. Indicate whether a solution of the product would be optically active or no not active and provide a simple reasoning to support your choice.
Need the last 2
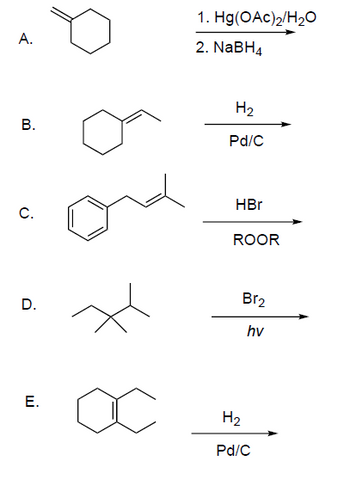
Transcribed Image Text:### Organic Chemistry Reactions
This image illustrates five different organic chemical reactions, each labeled from A to E, showcasing various transformation processes of organic compounds.
**A. Reaction:**
- **Starting Compound:** Cyclohexene
- **Reagents:**
1. Mercuric acetate (Hg(OAc)₂) in water (H₂O)
2. Sodium borohydride (NaBH₄)
- **Description:** This reaction likely involves an oxymercuration-reduction process, which is used to convert alkenes into alcohols with Markovnikov orientation, adding an -OH group to the more substituted carbon.
**B. Reaction:**
- **Starting Compound:** 1-methylcyclohexene
- **Reagents:**
- Hydrogen gas (H₂)
- Palladium on carbon (Pd/C)
- **Description:** This is a hydrogenation reaction, where the alkene is reduced by adding hydrogen across the double bond, converting the compound into an alkane.
**C. Reaction:**
- **Starting Compound:** Styrene
- **Reagents:**
- Hydrobromic acid (HBr)
- Organic peroxide (ROOR)
- **Description:** This reaction involves the radical addition of HBr, likely leading to an anti-Markovnikov addition due to the presence of the peroxide, resulting in an alkyl bromide.
**D. Reaction:**
- **Starting Compound:** 2,3-dimethylbutane
- **Reagents:**
- Bromine (Br₂)
- Light (hv)
- **Description:** This represents a free radical halogenation initiated by light, resulting in the substitution of a hydrogen atom with a bromine atom, forming a brominated alkane.
**E. Reaction:**
- **Starting Compound:** Cyclohexene
- **Reagents:**
- Hydrogen gas (H₂)
- Palladium on carbon (Pd/C)
- **Description:** Similar to Reaction B, this is a hydrogenation process where the alkene double bond is reduced to form cyclohexane by the addition of hydrogen.
Each reaction demonstrates fundamental concepts in organic chemistry related to transformations and functional group modifications using specific reagents and conditions.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY