
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
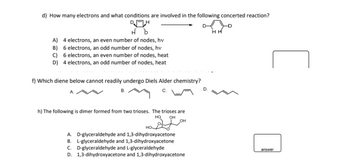
Transcribed Image Text:d) How many electrons and what conditions are involved in the following concerted reaction?
DH
A) 4 electrons, an even number of nodes, hv
B) 6 electrons, an odd number of nodes, hv
C) 6 electrons, an even number of nodes, heat
D) 4 electrons, an odd number of nodes, heat
f) Which diene below cannot readily undergo Diels Alder chemistry?
B.
h) The following is dimer formed from two trioses. The trioses are
HQ OH
OH
HO
A. D-glyceraldehyde and 1,3-dihydroxyacetone
B. L-glyceraldehyde and 1,3-dihydroxyacetone
C. D-glyceraldehyde and L-glyceraldehyde
D. 1,3-dihydroxyacetone and 1,3-dihydroxyacetone
HH
answer
[
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain [3,3] Sigmatropic Rearrangements ?arrow_forward5. When the diene below is dissolved in THF solvent to which a small amount of H₂SO4 has been added, cyclic products are formed that are isomeric with the reactant. Write a mechanism for the formation of ONE of these cyclic isomers, and include its structure. (In other words, you predict the product structure by going through the mechanism.) Include curved arrows in the mechanism to show bonding changes. yand H₂SO4 THF A cyclic isomer of the reactantarrow_forwardDraw the structures of Ferrocene, mono acetylated ferrocene, diacetylated ferrocene and order them from most polar to least polararrow_forward
- 8. Write an equation representing each of the following reactions: a. 1-Heptene+H2O (H+) b. 3-Heptene+H2 c. 2-Methyl-2-hexene+HCI d. 3-Methyl-1,4-cyclohexadiene+C12 e. 2,4-Heptadiene+Br2 f. 3-Methylcyclopentene+H2O (H+)arrow_forward4. You may know salicylic acid as an ingredient in anti-acne medication, or as a precursor to aspirin. Salicylic acid is also synthesized by the following reaction: ONa OH you 1. CO₂, heat 2. H₂O* a. Draw a reasonable arrow-pushing mechanism for the formation of the product. b. The challenge: Reach back to 2311 or general chemistry to propose why the ortho position is favored over para for this product.arrow_forwardIdentify the type of the following reaction: production of trans- and cis-2-butene from 2-chlorobutane a. E2 b. E1 c. SN1 d. SN2arrow_forward
- What reaction produces the following compound in good yield? a. Ethanoyl chloride + phenol in pyridineb. Acetic acid + phenol and sulfuric acidc. Acetic acid + benzened. Ethane + Sodium PhONa (Phenoxide) + Hot Sulfuric Acidarrow_forwardIn Diels-Alder experiment you refluxed the reactants for 30 minutes. What is refluxing and why is it necessary for some reactions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY