
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:D
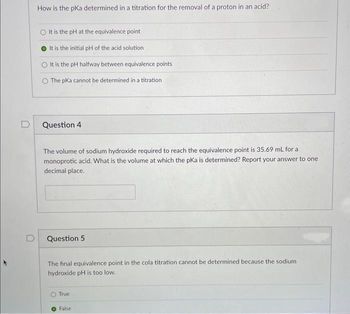
How is the pka determined in a titration for the removal of a proton in an acid?
It is the pH at the equivalence point
It is the initial pH of the acid solution
It is the pH halfway between equivalence points
O The pKa cannot be determined in a titration
Question 4
The volume of sodium hydroxide required to reach the equivalence point is 35.69 mL for a
monoprotic acid. What is the volume at which the pKa is determined? Report your answer to one
decimal place.
Question 5
The final equivalence point in the cola titration cannot be determined because the sodium
hydroxide pH is too low.
True
False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistry Questionarrow_forwardWhen 30 .00 mL of 0.1011 M HCl in 50 mL of deionized water is titrated against 0.09889 M NaOH, the pH increases. What is the volume (in mL) of NaOH required to reach the equivalence point and a pH of 7.00? Find the pH when the volume of NaOH added is 0.02 mL less than the volume required to reach the equivalence point. Find the pH when the volume of NaOH added is 0.01 mL less than the volume required to reach the equivalence point. Find the pH when the volume of NaOH added is 0.02 mL more than the volume required to reach the equivalence point. Comment on the significance of the changes in pH values in relation to the increments of sodium hydroxide added when going “through” the end point. Define equivalence point. For a weak base/strong acid titration, is the pH at the equivalence point <, >, or equal to 7?arrow_forwardWhat would be the molar ratio of cyanic acid (HCNO)/sodium cyanate (NaCNO) buffer having a pH of 4.80 ? [K a(HCNO) = 2.0 × 10 –4] 11.6 mol NaCNO to 1.0 mol HCNO 12.6 mol NaCNO to 1.0 mol HCNO None of the above 13.6 mol NaCNO to 1.0 mol HCNO 10.6 mol NaCNO to 1.0 mol HCNOarrow_forward
- Which statement is FALSE regarding the titration of a weak acid with a strong base? HA + OH" → A¨ + H2O O Before the equivalence point, the pH is due to a mixture of HA and A. O Past the equivalence point the pH is due to excess conjugate base, A". O Before strong base is added, the pH is due to the ionization of the weak acid, HA. O At the equivalence point, the pH is due to the hydrolysis of water by the conjugate base, A". O The pH at the equivalence point is basic.arrow_forwardConsider a titration between a strong acid and a weak base. What is the pH at the equivalence point? O PH 7arrow_forwardSelect all that are true for a titration at the equivalence point: Group of answer choices The number of moles of acid (ie H2SO4) added are the same as the number of moles of base (ie NaOH) added. The number of moles of hydronium ion added are the same as the number of moles of hydroxide ion added. The indicator is selected in order to have a color change when the equivalence point is reached.arrow_forward
- The graph below shows the titration of 9.15 g of a strong acid with 1.00 M NaOH. Which strong acid is it? tr pH 71.5 Volume 1.00 M NaOH Added (mL) O HNO3 O HI HCIO4 HBrarrow_forwardA 25. mL sample of a weak acid solution (KA= 4.3×10−4) is titrated with 0.20 M NaOH. 20.0 mL of NaOH are needed to reach the equivalence point What is the pH halfway to the equivalence point? 3.37 5.85 4.16 4.74arrow_forwardWhich one of the following titrations is expected to have a pH < 7 at the equivalence point?arrow_forward
- The following plot shows two titration curves, each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NAOH. 14 12 10 8 • d 4 b 2 20.0 40.0 60.0 80.0 100.0 mL of 0.100 M NAOH added 15) Which point a-d represents the equivalence point for the titration of a weak 15) acid? A) point a B) point b Opoint c D) point d 6. Hdarrow_forwardBewis a titra on curve of a weak acid with NaOH. What region of the titration curve can the volume of NaOH at equivalence point be located? O с B A 2 A B Vol. NaOH (mL) 6 C 8 10arrow_forwardAt which point of a titration curve, is the pH value of the solution equal to the pKa of the weak acid? O at the quarter equivalence point O at the equivalence point O at the half equivalence pointarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY