
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please provide the V
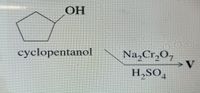
Transcribed Image Text:OH
cyclopentanol
Na,Cr,O,
H,SO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propanoic acid and methyl ethanoate are constitutional isomers. (c) оооо (d) CH3 оооо CH₂ OH Show how to distinguish between them by IR spectroscopy. (Use the Appendix.) (a) Which IR bands would one expect for propanoic acid? propanoic acid оооо 1200-1250 cm 1375 1450 cm 1450 1475 cm 1630-1820 cm -1 1200-1250 cm 1375 1450 cm 1450-1475 cm 1630-1820 cm 1375 1450 cm -1 -1 -1 -1 1200-1250 cm (b) Which IR bands would one expect for methyl ethanoate? -1 -1 1450 1475 cm -1 -1 1630-1820 cm -1 00000000 −1 CH₂-C 0000 methyl ethanoate If the IR spectrum shows a band at 1780 cm-¹, which isomer is it? 1700-1725 cm 1735-1800 cm 2720 cm -1 2500-3300 cm 2720 cm Which IR bands are unique to, i.e. not shared between, the two sets? -1 1700 1725 cm 2500-3300 cm -1 CH3 1735-1800 cm -1 -1 -1 2720 cm -1 2500-3300 cm -1 1700-1725 cm -1 1735-1800 cm -1 -1 -1arrow_forwardSelect the partial structure for a doublet integrating to 2H at 2.3 ppm. O=C-CH2-CH O-CH2-CH2 OC=C-CH₂-CH2 C-CH₂=CHarrow_forward(a) (c) 12.4 Which IR spectrum is consistent with an alkene? 100 100 80 80 60 60 40 40- 20 20 1500 1000 2000 2500 Wavenumber (cm¹) 2000 2500 Wavenumber (cm¹) % Transmittance 0. 4000 100 % Transmittance 80- 60 40- 20. 0. 4000 3500 3500 3000 3000 1500 1000 (b) (d) % Transmittance % Transmittance 0 4000 100 80 60- 40- 20 0. 4000 3500 3500 3000 3000 mm y 1500 2500 2000 Wavenumber (cm¹) 2000 2500 Wavenumber (cm) 1500 1000 1000arrow_forward
- Estimate the pKa values for the functional group classes represented by the given molecules. D 80 F3 $ 4 C R F OH V DOO OOO F4 % 5 35 T G F5 B 6 OH 10 Y F6 MacBook Pro H 15 Answer Bank NH3 & 7 N F7 U 50 J 25 8 I M DII F8 K ( 9 H 5 DD F9 O L I C=C—H O L F10 P > 2 i F11 { L IZ + 11 ? 1 F12 } ] delearrow_forwardPlease Calculate the dipole moment of Glycine molecule using partial charge and positions of atoms.arrow_forward2. HE=ACHE 6. Af=HCA2C 8. 7. Ag 9. 4. 10. 5. VO CH3 Br excess о Br CH₂ C-ce десез Br CHCHCH ce десез все CH2-CH2 CH3 не сез Az 10005 <30°с H₂ 2dtm 2506 0= C- CH₂ CH3 Suce₂ нзой CH3 за Сег ал сез а Во major product onlyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY