
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I ONLY NEED THE SOLUTION TO BE TRANSCRIBED INTO DIGITAL FORMAT, AT LEAST THE LETTERS SINCE THEY ARE NOT UNDERSTOOD, IT IS A SOLUTION FROM HERE BUT I DO NOT UNDERSTAND WHY YOU EXPERTS DON'T MAKE IT CLEAR, THAT'S WHY I TAKE ANOTHER CREDIT TO IMPROVE THE WORK
TRANSCRIBE THE SOLUTION IN DIGITAL FORMAT!
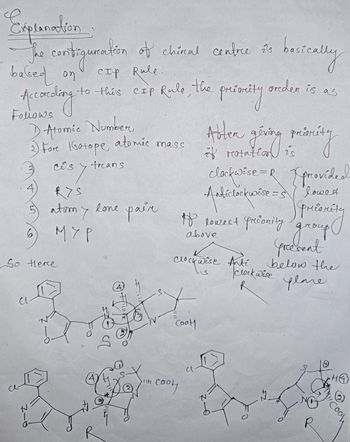
Transcribed Image Text:Expranation
The constiguration of chiral centre sis
it's
CIP Rile.
based on
According
to this CIP Rule, the
1 Atomic Number
3) Fore Gatope, atomic mass
-Z
cos
is y trans
RYS
So Herce
atomy
6) мур
R
lone pair
(4)
tute Co
соон
Clockwise Anti
Ꮗ
CooH
presoreity
Abter giving
givning minity
if rotatio
to lowest priority
above
Cl
basically
greden is as
Anticlockwise = 5
clockwise =R [ provided
lowest
priority
bitly
group
preesent
below the
plane
не
clockwise
33
HO
Cool
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amazon SNYS DMV - My DMV 14.udemy ClassMarker - Test NYC HEALTH +HOS... Which statement does the passage support? Question 15 of 190 Although most animals and some plants detect and respond to sound vibrations, the precise sensitivity that we call hearing is rare in the living world. It is highly developed only in birds and mammals, and the same operational system applies in all vertebrate auditory systems. Sound waves cause a liquid within the organism's auditory apparatus to vibrate. These vibrations are picked up by receptors that transmit signals to nerve cells. These cells then communicate the sound to the brain. Humans can hear vibrational frequencies of 20 to 20,000 cycles per second; cats respond to frequencies of up to 50,000 cycles per second; and porpoises and bats pick up frequencies of 100,000 cycles per second. O A. Ears are damaged by high frequencies. O B. Humans have the best hearing of all animals. O C. Cats and porpoises are adapted to hearing low frequencies. O D.…arrow_forwardStep 3 of 8 Sum the equations for d[H] and d[Br] and solve for [Br]. (Use the following as necessary: [Br2], [H], [H2], [HBr], k1, k2, k3, k4, and k5.) dt dt [Br] = K5 2k1 [Br.] 2k [Br2] ks Step 4 of 8 Using the equation for I from Step 2, solve for [H]. (Use the following as necessary: [Br], [Br2], [H2], [HBr], k1, k2, k3, k4, and k5.) dt ky[ Br][H,] kg[Br] + k4 [HBr] [H] = k2[Br][H2] k3[Br2] + k4[HBr] Step 5 of 8 Substitute the equation for [Br] from Step 3 into the equation for [H] from Step 4 and simplify. (Use the following as necessary: [Br2], [H2], k1, k2, k3, k4, and k5.) k, k5 [Br.] [H] k3[Br2] + k4[HBr] Submitarrow_forwardW AutoSave O Search (Alt+Q) Off ASSIGNMENT 24.docx - raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Lato v 18 - A A Aa v A No Spacing Heading 1 Heading : E Replace Normal Paste I U ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard Font Paragraph Styles Editing Voice Editor Reuse Files Question 18 Consider the following reaction mechanism: CH3OH(ag) + H*(ag) – CH3OH2*(ag) CH;OH2*(ag) + Br(ag) → CH3Br(ag) + H2O(1) Identify each species appropriately from the list below. Question 18 options: CH;Br(ag) CH;OH(ag) 1. reactant H2O(1) 2. product H*(ag) 3. intermediate Br(ag) 4. catalyst CH;OH2*(ag) Question 19 * Accessibility: Investigate D'Focus 0% 11 Page 2 of 16 1271 words English (Canada) ENG 4:32 PM O Type here to search 0°C Sunny W US 2022-04-26 11 近arrow_forward
- I dont understand. Could you rewrite better? I think its wrong.arrow_forwardI know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forwardas X Clas X ||| STE X © Ban X Jen Scho × Ban X CD www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNsikr7j8P3jH-liG_IZvpRqwiHv-fgOzocXR7H3QULJsrn-hH7iM_OKtS081EM1kmGkq29c dent Bookmarks 00 Duolingo - The worl... ▸ YouTube Mary G. Ross: Who... Zomberry Hero > Try Again Cha X O MATTER Finding the side length of a cube from its volume in liters Your answer is incorrect. 0.75 m Trial X Explanation A technical machinist is asked to build a cubical steel tank that will hold 475 L of water. Recheck 198 X Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. → # X $ " % d H 6 H Copy of Cause and... FORENSIC SCIENC... kh hp Bay x M & 7 Ⓒ2023 McGraw Hill LLC. All Rights Re C Cop X * 00 8 ( 9arrow_forward
- MyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X - со A ALEKS-Amber Bail www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd Gmail YouTube Maps MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc O CHEMICAL REACTIONS = Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 g ANAKKALE X S ? Email 4 Jessy Vseforestainty a jedan den so $45******arrow_forwardA student determined that the mass of four pennies was 8.7 grams. Use this information to calculate the number of pennies in a box that contains 2.5 pounds of pennies. Rouund your answer to the nearest whole number. For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).arrow_forwardAnswer correctly pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY