
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
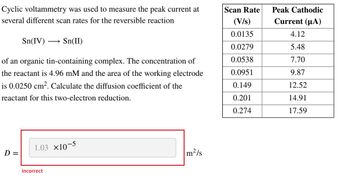
Transcribed Image Text:Cyclic voltammetry was used to measure the peak current at
several different scan rates for the reversible reaction
Sn(IV) → Sn(II)
of an organic tin-containing complex. The concentration of
the reactant is 4.96 mM and the area of the working electrode
is 0.0250 cm². Calculate the diffusion coefficient of the
reactant for this two-electron reduction.
D =
1.03 ×10-5
Incorrect
m²/s
Scan Rate
(V/s)
0.0135
0.0279
0.0538
0.0951
0.149
0.201
0.274
Peak Cathodic
Current (μA)
4.12
5.48
7.70
9.87
12.52
14.91
17.59
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Eºc cell Ecell = 0.135 V for the reaction 312(s) + 5Cr₂O7²-(aq) + 34H*(aq) →→ 6103¯(aq) + 10Cr³+ (aq) + 17H₂O What is Ecell if [Cr₂O7²-] = 0.036 M, [H*] = 0.28 M, [103] = 0.00027 M, and [Cr³+] = 0.0015 M? Varrow_forwardCalibration of a glass electrode gave a reading of 139.3 mV with a 0.05 m potassium hydrogen phthalate buffer standard ( pH = 4.015), and a reading of -160.7 mV with a 0.01 m borax buffer standard (pH = 9.139), both measured at 30 °C. What is the observed slope (mV/pH unit) of the calibration curve? slope observed What is the theoretical slope at 30 °C? = slope theoretical pH : What is the pH of an unknown that gives a reading of 42.5 mV with this electrode at 30 °C? = = mV/pH mV/pHarrow_forwardCalculate the cell potential (in V) of the following cell with transference at 25°C: Pb(s) PbSO4(s), CuSO4(m = 0.2, y = 0.110) CuSO4(m = 0.02, y = 0.320), PbSO4(s) Pb(s) The transport number of Cu²+ is 0.370. Express your answer in three significant figures.arrow_forward
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY