
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Match each of these titration curves (listed in order from left-to-right, Curve I, Curve II, Curve III) to its correct titration:
Anwer choices
titration of NH3 with HBr
titration of HI with KOH
titration of HF with NaOH
titration of RbOH with HNO3
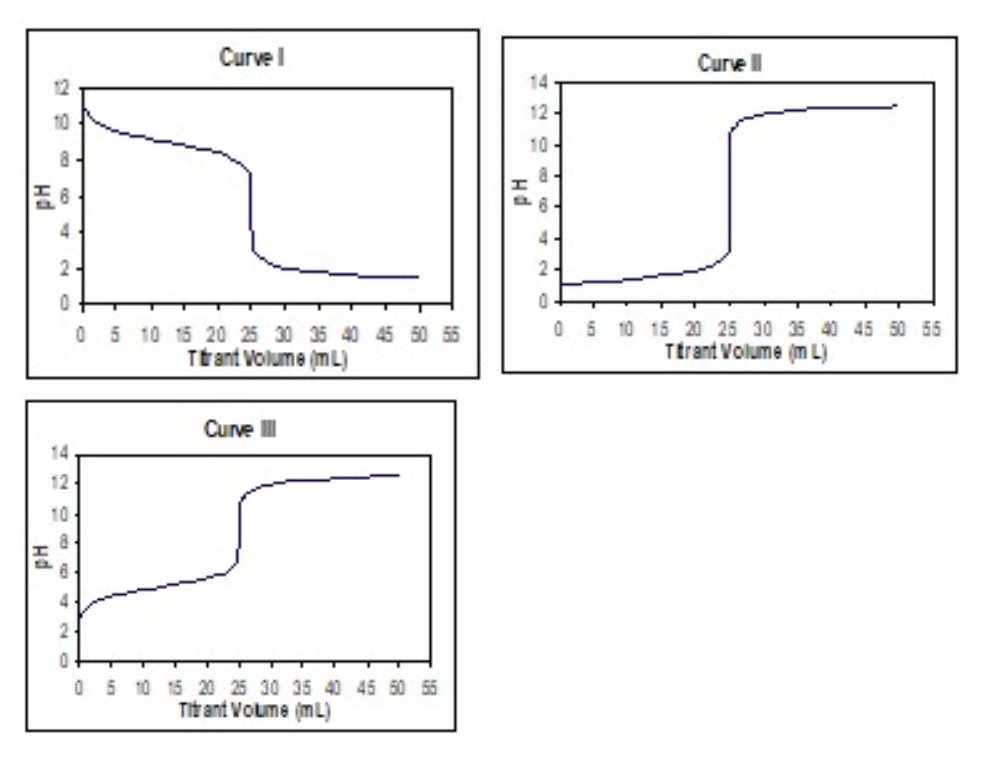
Transcribed Image Text:Curvel
Curve l
12
10
10
8.
2.
05 10 15 20 25 30 35 40 45 50 55
Ttant Volume (mL)
5 10 15 20 25 30 35 40 45 50 55
Ttrant Volume (m L)
Curve II
14
12
10
2-
5 10 15 20 25 30 35 40 45 50 55
Titr ant Voume (mL)
нd
нd
нd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the function of indicator in a titration? Edit View Insert Format Tools Table 12pt ✓ Paragraph BIU IU AT²V 2 T² : Carrow_forwardA titration of 40.0 mL of a sulfuric acid (H₂SO₄) solution of unknown concentration with a standardized 1.55 M Ba(OH)₂ solution requires 23.0 mL to reach the second equivalence point. What is the concentration of the H₂SO₄ solution?arrow_forward1.00 x 10-² A student was titrating a solution of HC4H7O₂ with a Sr(OH)2 solution. Determine the pH at a particular point in the titration. Do this by constructing a BCA table and determining the pH. Complete Parts 1-2 before submitting your answer. Before (mol) Change (mol) After (mol) NEXT > 40.0 mL of a 0.200 M HC4H7O2 solution was titrated with 100 mL of 0.100 M Sr(OH)2 (a strong base). Fill in the ICE table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of water in the reaction. 0 -1.00 x 10-² 1 HC4H-O₂(aq) + 0.100 1.20 x 10-² 0.200 OH-(aq) -1.20 x 10-² 2.00 x 10-³ 2 2.00 x 10-² H₂O(l) + -2.00 × 10-³ -2.00 x 10-² C4H7O₂ (aq) 8.00 x 10-³ RESET -8.00 x 10-³arrow_forward
- A student performed titration to determine the concentration of citric acid with potassium permanganate solution. The net ionic equation is as follows: 5C6H8O7 + 18MnO4- + 54H+ → 30CO2 + 47H2O + 18Mn2+ The concentration of potassium permanganate soluiton is 0.266 M. The volume of lemon juice is 25.00 mL. The titration consumed 17.6 mL of potassium permanganate solution to reach the endpoint. Determine the moles of citric acid in the lemon juice sample.arrow_forwardEach graph below represents a titration involving solutions of two of the following substances: HC2H3O2, HNO3, CH3NH2 and NaOH. Which graph best represents HC2H3O2 being titrated using NaOH? Which graph best represents HC2H3O2 being titrated using CH3NH2? Which graph best represents HNO3 being titrated using NaOH? Which graph best represents HNO3 being titrated using CH3NH2? Which graph has the lowest pH at the equivalence point? Which graph has the equivalence point pH that is equal to 7?arrow_forwardTitrating ascorbic acid with iodine is what type of titration? HO. HO. + 2 HI HO 3x-3x= h + HO HO OH Iodine Ascorbic Acid Dehydro-Ascorbic Acid O An acid-base neutralization titration, because iodine is a strong acid OA redox titration, because iodine acts as an oxidizing agent OA redox titration, because iodine acts as a reducing agent An acid-base neutralization titration, because ascorbic acid is a weak basearrow_forward
- Two 25.0-mL. samples of unknown monoprotic weak acids, A and B, are titrated with 0.100 M NAOH solutions. The titration curve for each acid is shown below ACID A ACID B 14 14 12 12 10 10 10 15 20 25 30 35 40 45 30 5 10 15 20 25 30 33 40 45 30 Vohume of NaOH added (ml) Volume of NaOH added (ml ) Part A Which of the two weak acid solutions is less concentrated? B A. Submit Bequest Answerarrow_forwardRank the following titrations from the endpoint with the lowest pH to the endpoint with the highest pH: 5. 2.0 M C3H;N titrated with 1.0 M HCI 1.0 M NHẠC1 titrated with 2.0 M NaOH 2.0 M NaCIO titrated with 1.0 M HCI 1.0 M HNO2 titrated with 2.0 M NaOH 2.0 M NaHCOO titrated with 1.0 M HCIarrow_forward40 mL of LiOH are titrated to the equivalence point with 65 mL of 1.2 x 10-2 M HNO3. Find the concentration (molarity) of the LiOH solution.arrow_forward
- Calculating the pH of a weak acid titrated with a strong base 0/5 Izabella An analytical chemist is titrating 127.4 mL of a 0.4200M solution of nitrous acid (HNO 2) with a 0.2400M solution of KOH. The pK of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 260.9 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places. pH = 0 ☑ G 000 18 Ar Barrow_forwardThe pKa of hypochlorous acid is 7.530. A 51.0 mL solution of 0.122 M sodium hypochlorite (NaOCI) is titrated with 0.323 M HCI. Calculate the pH of the solution after the addition of 7.44 mL of 0.323 M HCl. pH = Calculate the pH of the solution after the addition of 20.0 mL of 0.323 M HCl. pH = 7.73 pH = 2.475 Calculate the pH of the solution at the equivalence point with 0.323 M HCI. & TOOLSarrow_forwardWhen a 20.9 mL sample of a 0.387 M aqueous hydrofluoric acid solution is titrated with a 0.372 M aqueous barium hydroxide solution, what is the pH at the midpoint in the titration?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY