
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:lyprotic Acids
What Is The PH Of A H2PO4-/HPO4^2- Buffer In Which... | Chegg.com
titration curve for the titr
6-Calculate the pH of a H2PO4-/ HPO42- buffer in which the concentration of
the weak acid component is 0.130M and the concentration of the conjugate base
is 0.026M
H3PO4
Ka
7.1 x 103
6.3 x 108
4.5 x 10-13
1st
2nd
Brd
O 7.20
6.50
7.90
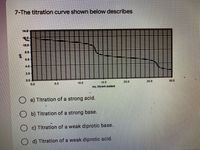
Transcribed Image Text:7-The titration curve shown below describes
14.0
10.0
4.0
4.0
20
0.0
10.0
15.0
20.0
250
mL titrant added
O a) Titration of a strong acid.
b) Titration of a strong base.
c) Titration of a weak diprotic base.
O d) Titration of a weak diprotic acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the graph below. What type of titration does it represent? 12 8 2 0+ 0 10 20 30 40 50 60 volume added (m L) A weak acid titrated with a strong base. A weak base titrated with a strong acid. A strong base titrated with a strong acid. A strong acid titrated with a strong base. 10arrow_forwardDetermine the pH during the titration of 14.4 mL of 0.130 M perchloric acid by 7.26E-2 M sodium hydroxide at the following points:a. Before the addition of any sodium hydroxide b. After the addition of 12.9 mL of sodium hydroxide c. At the equivalence point d. After adding 33.5 mL of sodium hydroxidearrow_forwardWhich titration curve could describe the titration of a solution of HCI by addition of a solution of KOH? 'a.¤ pH 7 'b.x vol KOH added 'C.¤ pH 7 0 vol. KOH added a pH 7 0 vol. KOH added a 'd.¤ 'e.¤ pH 7 X 0 vol. KOH added a pH 7 0 vol. KOH added aarrow_forward
- What is the difference between the endpoint and the equivalence point of a titration? A. The endpoint cannot be seen, the equivalence point requires an indicator B. The equivalence point is the halfway point of the titration, the endpoint is the end of the titration. C. The endpoint is halfway through the titration and the equivalence point is the end of the titration. D. The endpoint is when a color change occurs due to the addition of an indicator that indicates the equivalence point has been reached.arrow_forwardA 25.0 mL sample of a saturated calcium hydroxide solution is titrated with 0.023 M HCl and the equivalence point is reached after 38.0 mL of titrant are dispensed. What is the concentrationof the hydroxide ion?arrow_forward8. Consider the titration of a sample of a weak acid with a strong base (titrant). What is the relative pH at the equivalence point? Group of answer choices A) greater than 7.00 B) less than 7.00 C) exactly 7.00arrow_forward
- What is the pH of a buffer made by combining 45 ml of 0.15M nitrous acid and 20 ml of 0.175 M sodium nitrite a. 3.43 b. 3.64 c. 3.28 d. 3.06arrow_forwardPlease don't provide handwritten solution ....arrow_forwardAt which point in the titration curve below is a buffer present? C Volume titrant added (mL) A. Ο Ρoint A В. О Рoint B C. O Point C D. O Point D E. OA buffer is not present at any point during this titration.arrow_forward
- The graph below represents the titration curve of a A. 1 M strong acid with 1 M strong base B. 1 M weak base with 1 M strong acid C. 1 M strong base with 1 M strong acid D. 1 M weak base with 1 M weak acid E. 1 M weak acid with 1 M strong basearrow_forwardWhich graph best represents the titration of sodium hydroxide with hydrochloric acid? O a. 14 volume HCl added Ob. volume HCl added Oc. 14 volume HCl added Od. 14 volume HCl added Hd Hd Hd Hdarrow_forwardConsider Titration of 25 mL of .1 M CH3CO2H With .1 M NaOH. Calculate The pH After Adding The Following Volumes of NaOH: a. 0 mL b. 12.5 mL. c. 20 mL d. 25 mL. e. 37.5 mL. I do know the answers, I just don't know the way to get to these answers. a. pH = 2.87 b. pH = 4.74 c. pH = 5.34 d. pH = 8.72 e. pOH = -log(.0192 M) = 1.72 pOH 14-1.72 = 12.28 pH (I just know the last steps to the answer for part e, but don't know all the other steps to get to answer).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY