
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
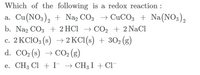
Transcribed Image Text:Which of the following is a redox reaction :
a. Cu(NO3), + Na, CO3 → CuCO3 + Na(NO3),
b. Na2 CO3 + 2 HCl → CO2 + 2 NaCl
c. 2 KCIO3 (s) → 2 KC1(s) + 302 (g)
d. CO2 (s) → CO2 (g)
S
e. CH3 Cl + I → CH3I + Cl¯
→ CH3 I + Cl-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the spectator ions. Sr(OH)2+K2S=2KOH+SrSarrow_forwardOctane (C8H18), a component of gasoline, reacts with oxygen to form carbon dioxide and water. Write the balanced chemical reaction for this process by passing a single piece of paper around your group, having each group member complete the next logical step. As you each complete your step, explain your reasoning to the group.arrow_forwardLighters typically contain butane (C4H10), which has a molar mass of 58.12 g/mol. Consider that there may have been a mixture of hydrocarbons present in the lighter. If the experimentally obtained molar mass of this mixture was 56.1 g/mol, what other hydrocarbons may have been present? Choices include: CH4 (methane), C2H6 (ethane), C3H8 (propane), C5H12 (pentane), C6H14 (hexane), C7H16 (heptane), or C8H18 (octane). Explain.arrow_forward
- Morphine sulfate used for severe pain relief is an opiate alkaloid isolated from the plant Papaver somniferum. In the central nervous and gastrointestinal systems, this agent has widespread effects including analgesia, anxiolysis, euphoria, sedation, respiratory depression, and gastrointestinal system smooth muscle contraction. Morphine sulfate has 34 Carbon atoms, 40 atoms of Hydrogen, 2 Nitrogen atoms, 10 Oxygen atoms, and 1 atom of Sulfur. What is the Chemical formula of this compound:arrow_forwardWrite the complete balanced equation for the combustion of octane (C8H18), which is one of the most important components in gasoline.arrow_forwardb. Given the chemical reaction 5 S(s) + 6 02 (g) → 3 SO2 (g) + 2 SO3 (g) Which of the following flux relationships is True? i. NO2 = 2 NSO2 ii. NSO2 = 3/2 NSO3 iii. NO2 = -1/2 NSO2 iv. NSO2 = -2/3 NSO3arrow_forward
- Dragonium (Dr) is an element only found in the scales of dragons. It is known to exist only in the oxidation state of +3, and it is a metal. After dragons go for their morning swim in the Pacific Ocean, their scales change color due to the formation of a chlorine salt of dragonium which crystallizes out with four water molecules per each dragonium atom. Select the name of this compound.arrow_forwardWhich copper ore contains the highest percentage of copper by weight? Mathematically document your answer (Cu=63.5; S=32.0; O=16.0; H=1.00; C=12.0) Brochantite CuSO4 x 3 Cu(OH)2 or Azurite 2 CuCO3 x Cu(OH)2 ?arrow_forwardCalculate the molecular weight for Al2 (SO4 )3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY