
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
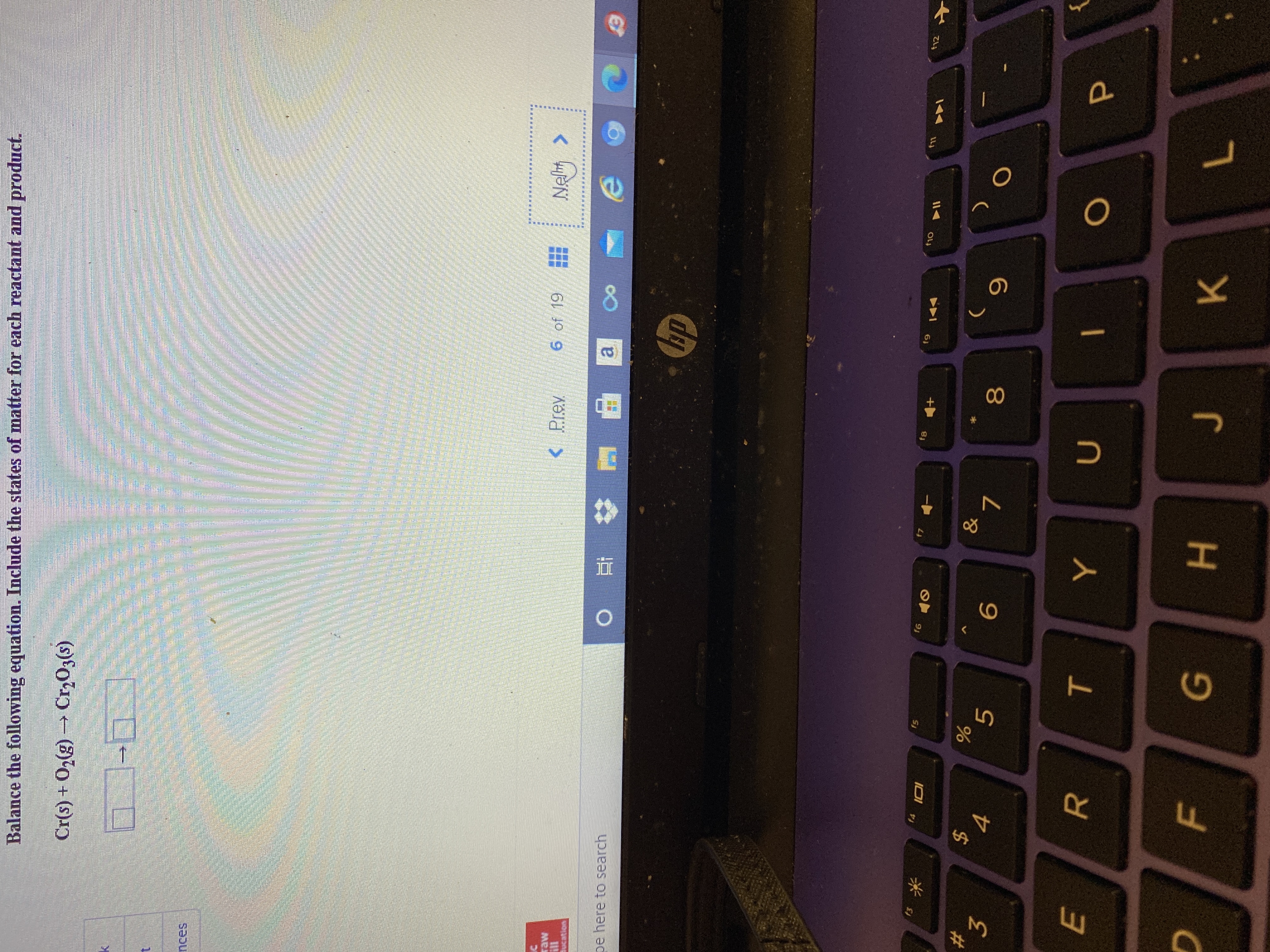
Transcribed Image Text:Cr(s) + O2(g)→ Cr,O3(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the species being oxidized and reduced in the following reaction: Cr* + Sn4+→Cr3+ + Sn2+ Cr*: reduced , Sng*: oxidized Neither is being oxidized or reduced Cr*: oxidized, Sna*: reduced O O Oarrow_forwardDescribe how the following changes are brought about: (i) Pig iron into steel. (ii) Zinc oxide into metallic zinc. (iii) Impure titanium into pure titanium.arrow_forwardIndicate whether each species is a reactant, product or spectator. Dilute sulfuric acid is added to zinc to form hydrogen gas. 2H+(aq) + SO42-(aq) + Zn(s) → H2(g) + Zn2+(aq) + SO42-(aq)arrow_forward
- 9. Write the balanced equation for the following reactions a. C6H10(g) + O2(g) ⟶⟶ CO2(g) + H2O(l) b. C11H18O7N5 + O2 → CO2 + H2O + NH3arrow_forwardWhy was this wrong and how do I get the right answer?arrow_forwardThe reaction between ammonia and oxygen is given below:2 NH3(g) + 2 O2(g) N2O(g) + 3 H2O(l)We therefore know that which of the following reactions can also occur? 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) N2O(g) + 3 H2O(l) 2 NH3(g) + 2 O2(g) 4 NO(g) + 6 H2O(g) 4 NH3(g) + 5 O2(g) None of the Abovearrow_forward
- Can you please solve this on notebbok.arrow_forwardGiven the following reaction: 3 NiCl2 + 2Na3PO4 → Ni3(PO4)2 +6NaCl, what is the mole ratio for NiCl2 to NaCl. Single line text.arrow_forwardWhich of the following used to be more precious than gold or silver, due to difficulties in refining it? Aluminum O Iron O Tin O Copper Zincarrow_forward
- Write the balanced chemical equation for the fermentation of sucrose (C12H22O11) by yeasts in which the aqueous sugar reacts with water to form aqueous ethanol (C2H5OH) and carbon dioxide gas.arrow_forward6arrow_forward3. What mass of oxygen is produced if 22.7 mol of carbon dioxide is consumed in a controlled photosynthesis reaction? 6CO₂(g) + 6H₂O(l) → C6H12O6(s) + 60₂(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY