
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
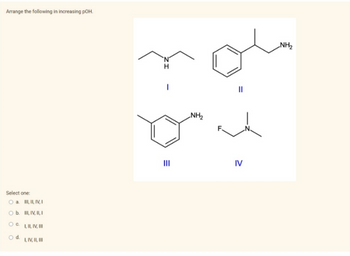
Transcribed Image Text:Arrange the following in increasing pOH.
Select one:
O a. III, II, IV, I
O b.
III, IV, II, I
O C.
I, II, IV, III
O d.
I, IV, II, III
I
E
|||
NH₂
IV
NH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is true about the reaction below? a.) PF; K > 1 b.) PF; K < 1 c.) RF; K < 1 d.) RF; K > 1arrow_forwardWhich of the following compounds will dissociate into the most ions? A. NaCl B. H2SO4 C. Fe3(SO4)2 D. MgCO3 E. None of the abovearrow_forwardplease don't provide handwrittin solution....arrow_forward
- 10. The ionization constant for HNO2 is 2.70 x 10-6 at 25 oC. Determine the standardfree energy change for the ionization: NO2- (aq) + H2O (l) ------ HNO2 (aq) + OH- (aq)A. 31.77 kJ/mol B. 38.10 kJ C. 58.10 kJ/mol D. 40.20 kJ/mol E. 48.10 kJ/molarrow_forwardWhen a mixture containing cations of Analytical Groups I–III is treated with H 2S in acidic solution, which cations are expected to precipitate? a. Analytical Group I only b. Analytical Group II only c. Analytical Groups II and III d. Analytical Group III only e. Analytical Groups I and IIarrow_forwardThe elements sodium, aluminum, and chlorine are in the same period.. (a) Which has the greatest electronegativity?. (b) Which of the atoms is smallest?. (c) Write the Lewis structure for the simplest covalent compound that can form between aluminum and chlorine. (d) Will the oxide of each element be acidic, basic, or amphoteric?arrow_forward
- 1. In which species does bromine have an oxidation number 0? 2. Balanced molecular equation for complete neutralization of H2SO4 by KOH in aq solution is?arrow_forward(M) (M) (M) (M) (M) M 1req 1req 1req 1req 2req 2req 1req M M (M ICHIG, a. PbCl, lead(II) chloride O correct Oplumbous chloride Olead(IV) chloride Olead chloride b. CuS, copper(II) sulfide Ocorrect Ocopper sulfide Ocopper(II) sulfite Ocopper sulfite c. PbBr2, plumbic bromide Ocorrect Olead(IV) bromide Olead(II) bromide Olead bromide d. LiI, lithium(I) iodine correct lithium iodide Olithium iodine lithium(I) iodide e. NiO, nickel(I) oxide correct nickelium oxide nickel oxide nickel(II) oxide Show Hint [Review Topics] [References] Learning Previous N Saarrow_forwardYou have been contracted to determine how different salts affect the pH of water. Which of the solids in the following set should you test to investigate for the effects of anions on pH? O RbF O KCIO O AIBr3 O Rb2SO3 O CrCl3arrow_forward
- The solubility products for a series of iodides area. CuI Ksp = I X 10-12b. AgI Ksp = 8.3 X 10-17c. Pbl2 Ksp = 7.1 X 10-9d. BiI3 Ksp = 8.1 X 10-19List these four compounds in order of decreasing molar solubility ina. water.b. 0.10 M NaI.c. 0.010 M solution of the solute cation.arrow_forward1:26 PM Online teaching and X now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take&takeAssignmentSessionLocator ags Review Topics] References Use the References to access important values if needed for this question. The acid ionization constant for Cu(H20)6 (aq) is 1.0x10. Calculate the pH ofa 0.0208 M solution of 2+ Cu(NO3)2 pH = Submit Answer Retry Entire Group 4 more group attempts remaining Visited Next O verizonarrow_forwardProvide conditions for the following reactions (images attached)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
