
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Determination of phosphorus in blood serum gave results of 4.39, 4.43, 4.62, 4.46, and 4.50 ppm P. Determine whether the 4.62 ppm result is an outlier or should be retained at the 95% confidence level.
Q =
Q crit =
Decision: Retain or Reject
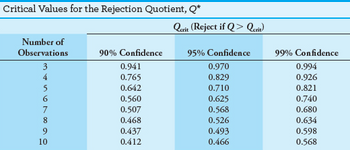
Transcribed Image Text:Critical Values for the Rejection Quotient, Q*
Qcrit (Reject if Q> Qcrit)
Number of
Observations
3
90% Confidence
95% Confidence
99% Confidence
0.941
0.970
0.994
456789
0.765
0.829
0.926
0.642
0.710
0.821
0.560
0.625
0.740
0.507
0.568
0.680
0.468
0.526
0.634
0.437
0.493
0.598
10
0.412
0.466
0.568
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- In a recently published case control study, the researcher reported an odds ratio of 3.55 (95% CI 1.88-5.4). This indicates that the OR is significant and the associated p=value is less than 0.05. A. TrueB. False please explain the reasoning behind the answer, thank you!arrow_forwardUsing this dehydration method, any error less than 10% is acceptable. A. If your percent error is less than 10% discuss what portions of the experiment were most important to achieve accurate results My percent error was 3.32% so answer the question abovearrow_forwardONLY ANSWER 3arrow_forward
- How does one measure Km using a Lineweaver-Burk plot? 1. By determining the reciprocal of the y-intercept 2. By determining the negative reciprocal of the x-intercept 3. By determining the slope and multiplying it by kcat 4. By determining the saturation pointarrow_forwardDon't use Ai and chatgpt. Answer in step by step with explanation.arrow_forwardWHAT IS THE NULL HYPOTHESIS OF A CHI-SQUARED TEST? • WHAT DO YOU DO IF THE P- ● VALUE IS EXACTLY 0.05? ●arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON