
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Part B specifically please :)
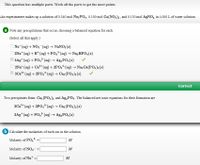
Transcribed Image Text:This question has multiple parts. Work all the parts to get the most points.
An experimenter makes up a solution of 0.340 mol Nag PO4, 0.100 mol Ca(NO3)2, and 0.130 mol AgNO, in 4.000 L of water solution.
a Note any precipitations that occur, choosing a balanced equation for each.
(Select all that apply.)
O Na+ (ag) + NO3 (ag) → NANO3 (s)
O 2Na* (ag) + H* (ag) +PO, (ag) → Na, HPO4(s)
3Ag+ (ag) + PO (ag) → Ag3 PO4(s)
3-
2Na* (ag) + Ca?+ (aq) + 2PO,* (ag) → Na2 Ca(PO4)2(s)
3Ca2+ (aq) + 2PO,* (ag) → Cas (PO4)2 (s)
Correct
Two precipitates form: Caz (PO, )2 and Ag; PO4. The balanced net ionic equations for their formation are
3Ca+ (ag) + 2PO, (ag) → Cas (P2(s)
3Ag* (ag) + PO,* (aq) A83PO4(s)
b Calculate the molarities of each ion in the solution.
Molarity of PO,
M
Molarity of NO3
M
Molarity of Na+ =
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could you explain how you got R for (c)?arrow_forwardPart D One of these liquids is used as a "blowing agent" in the manufacture of polystyrene foam because it is so volatile. Which liquid would you expect to be used as a blowing agent? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help Pentane is used as the "blowing agent" because it has a boiling point and is Ethylene glycol volatile low high Submit Request Answer < Return to Assignment Provide Feedback 6:16 PM O 4) 12/12/2021 amazon B. Mastering. Spotify Free 65°Farrow_forwardSimplify by reducing to the fewest number of termsa(b/a)arrow_forward
- (a) dispersion force Dispersion forces are (Very weak),(Very strong) electrostatic attractions that occur due to the random electronic motion within all substances,(excluding),(including) those that are nonpolar. When the electrons within a molecule or atom are distributed asymmetrically about the nucleus, that molecule or atom will adopt a (temporary),(permanent), (induced),(instantaneous) dipole. The presence of this dipole can then distort the electrons of a neighboring atom or molecule, producing an (induced),(instantaneous) dipole. These two rapidly fluctuating dipoles thus result in a brief electrostatic (attraction),(Repulsion) between the two species. These forces are stronger in (Larger and heavier),(Smaller and lighter) atoms and molecules. For example, dispersion forces between (Fluorine),(iodine) molecules will be stronger than dispersion forces between (Fluorine),(iodine) molecules. (b) dipole-dipole attraction A dipole-dipole force is the electrostatic attraction…arrow_forwardPeardeck Exarcase use #5, Given whats below find all [],'s and calculote K [], O.100 Charge 0,010 Students, draw anywhere on this slide! Pear Deck Interactive Slide Do not remove this bar Slide 1/1arrow_forwardSelect one for each boxarrow_forward
- I want to know all the resonance structures Example Anisole Banzaldehyde Is there any more. Please provide all the resonance structures that I need to know for organic chem?arrow_forward1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?arrow_forwardH 1) NANH2 H3C 2) (CH3)2CHCH,Br 3) Na, NH3() || II IV V. A) B) C) D) E) I II III IV V A В C O Earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY